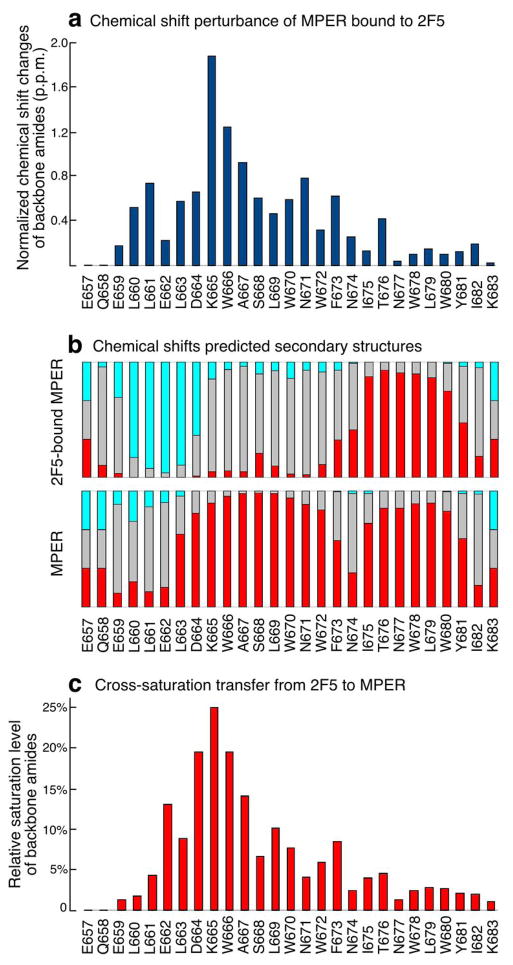
Figure 4. NMR characterization of the HxB2 MPER segment upon 2F5 Fab ligation.
(a) MPER backbone amide chemical shift changes upon binding to 2F5 Fab. The chemical shift values of both amide 15N and 1H are normalized as ((DH2 + (DN/5)2)1/2. (b) Secondary structures predicted from MPER chemical shifts by TALOS+. The red and light blue bars indicate probabilities of helical and beta-strand conformations respectively whereas grey bars represent unstructured regions. (c) Signal reduction observed in 2D-labeled MPER backbone amide peaks as transferred from 1H-saturated 2F5 Fab molecule to the MPER.