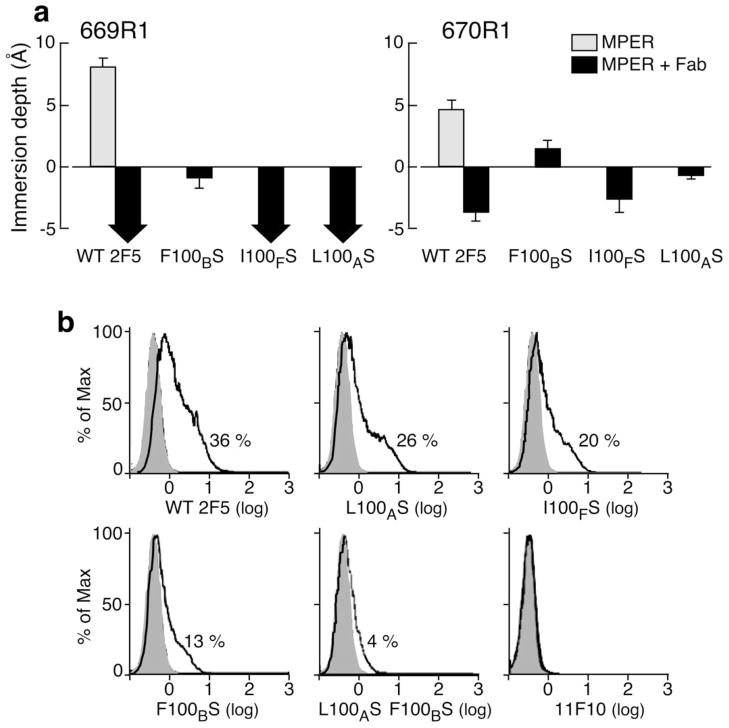
Figure 6. MPER membrane immersion depth changes following 2F5m ligation and their relationship on to trimeric envelope protein binding.
(a) Wild type and mutant 2F5 Fabs-induced membrane immersion depth changes of MPER R1 residues by EPR. Depth values of unligated MPER residues are indicated with gray columns. The values of MPER peptides in complex with wt 2F5 and 2F5m Fabs are shown as black columns. Depth values between −5 Å to 0 Å and larger than 0 Å correspond to lipid headgroup region and acyl chain region, respectively. The precise values of residues exposed to aqueous phase (depth <−5 Å) cannot be determined experimentally and are thus indicated by black arrows. (b) wt 2F5 and 2F5m binding to HIV-1 ADA envelope trimer expressed on 293 T cells. Cells transfected with ADA GP160 or empty vector were incubated with the designated antibody (5 μg ml−1) for 2h at RT followed by phycoerythrin-conjugated goat anti-human secondary antibody. Histogram indicates relative % cell staining by each antibody, respectively, after subtraction of background staining against mock transfected cells. Note the absence of cell staining by 11F10 even at the concentration of 200 μg ml−1.