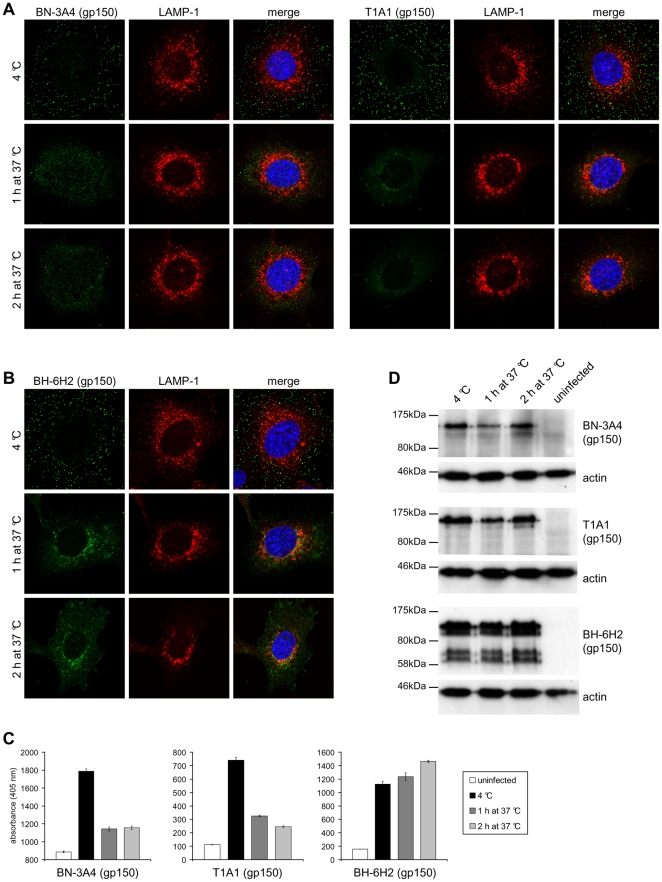
Figure 6. Antigenic changes in gp150 during virus entry.
(A) NMuMG cells were incubated with MuHV-4 (3 p.f.u./cell, 2h, 4°C), washed, and then either fixed immediately or first further incubated (1h and 2h, 37°C) to allow virion endocytosis. The cells were then stained with the gp150-specific mAbs BN-3A4 and T1A1 (green). BN-3A4 (IgG1) recognizes an epitope in the gp150 N-terminal (amino acid residues 1-150), T1A1 (IgG2a) an epitope in the central (residues 150-250). (B) Cells were infected and processed as in (A) but stained with mAb BH-6H2 (IgG2b), which recognises an epitope in the gp150 C-terminal (residues 301-450) extracellular domain. (C) Cells were infected and processed as in (A). Infected cells and uninfected control cells were then incubated with the gp150-specific mAbs BN-3A4 (IgG1), T1A1 (IgG2a), and BH-6H2 (IgG2b). Bound antibody was detected with alkaline phosphatase-conjugated secondary antibodies and incubation with p-nitrophenyl phosphate substrate. The bars show mean ± SEM values from 6 wells. The experiment shown is representative of two equivalent experiments. (D) Cells were infected and processed as in (A). Lysates of infected cells and uninfected control cells were then analyzed by immunoblot with the gp150-specific mAbs BN-3A4 (IgG1), T1A1 (IgG2a), and BH-6H2 (IgG2b). Detection of actin served as loading control.