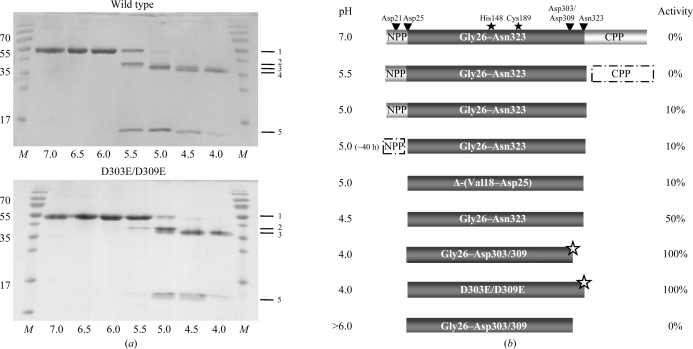
Figure 1.
Activation intermediates of legumain. (a) pH-dependent autoproteolytic processing shown by SDS–PAGE. SDS–PAGE of wild-type (wt) prolegumain and D303E/D309E prolegumain after incubation at the indicated pH values (310 K, 20 h). Lane M, molecular-weight marker (labelled in kDa); band 1, prolegumain (no cleavage); band 2, C-terminal propeptide cleaved (cleavage after Asn323); band 3, N- and C-terminal propeptides cleaved (after Asp25 and Asn323); lane 4, N-terminal propeptide cleaved and additionally processed at the C-terminus (Asp25 and Asp303/309); band 5, C-terminal propeptide. Band 4 was not observed for the D303E/D309E double mutant. (b) Schematic representation of autolytic cleavage intermediates. Activity is expressed as Bz-Asn-pNA turnover normalized to that of super-activated legumain. Autocatalytic cleavage sites are indicated by arrows. Δ-(Val18–Asp25) refers to the N-terminal truncation variant and D303E/D309E to the double mutant with disrupted C-terminal cleavage site. The variant at the bottom (pH > 6) illustrates irreversible inactivation of super-activated legumain after exposure to neutral pH. NPP, N-terminal propeptide (Val18–Asp25); CPP, C-terminal propeptide (Asp324–Tyr433); grey stars, super-activated legumain; filled stars, catalytic His148 and Cys189 residues.