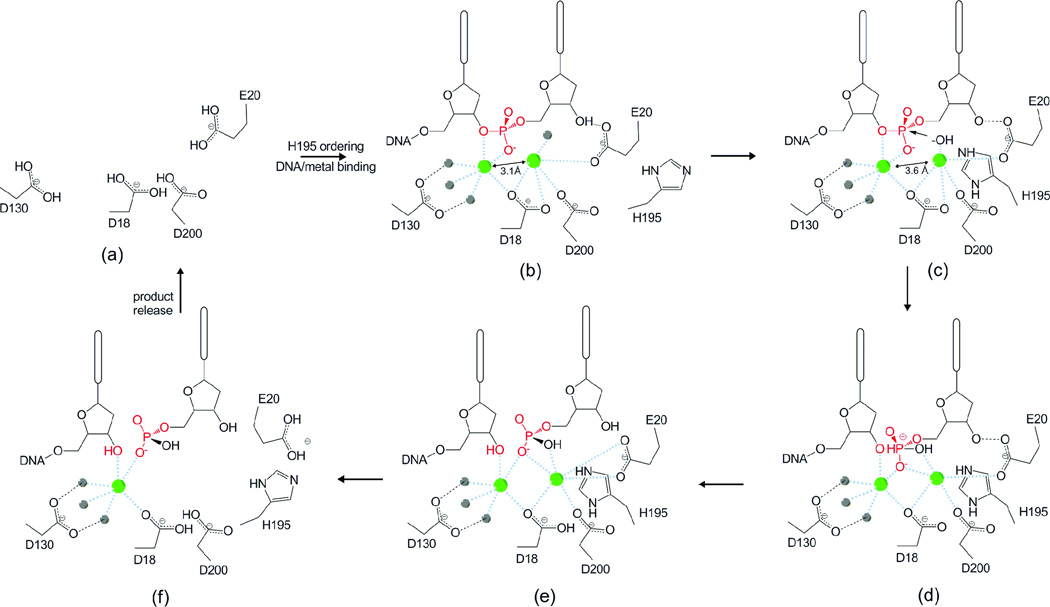
Figure 4. Proposed mechanism of the TREX1 exonuclease.
(a) In the absence of DNA and metal binding H195 in the active site is disordered. (b) Binding of DNA and metal ions (green spheres) orders residue H195 along with (c) a shift of divalent metal ions to inter-atomic spacing of ~3.6 Å. H195 contributes to deprotonation of a water molecule (gray sphere) above divalent metal ion a. (d) The activated nucleophile attacks the scissile phosphate (highlighted in red). The transition state (e) is stabilized by the divalent ions. Dissociation of the cleaved nucleotide monophosphate product and DNA may be facilitated by rotation of amino acids E20 and H195 away from the active site (f) allowing for the release of divalent metal and product from the enzyme in a distributive fashion.