Abstract
Background
To examine whether depression is associated with pre-existing hypertension or pregnancy-induced hypertension in a large sample of women attending a university-based obstetrics clinic.
Methods
In this prospective study, participants were 2398 women receiving ongoing prenatal care at a university-based obstetrics clinic from January 2004 through January 2009. Prevalence of depression was measured using the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) criteria based on the Patient Health Questionnaire-9 as well as the self-reported use of antidepressant medication. Evidence of pre-existing hypertension, pregnancy-induced hypertension and preeclampsia/eclampsia was determined by obstetrician ICD-9 codes. Logistic regression was used to quantify the association between hypertension in pregnancy and antenatal depression.
Results
After adjusting for sociodemographic variables, chronic medical conditions, smoking and prior pregnancy complications, women with pre-existing hypertension had an increased risk of Any Depression (minor, major, use of antidepressants) (OR = 1.55, 95% CI 1.08, 2.23) and Major Depression and/or use of antidepressants) (OR = 1.65, 95% CI 1.10, 2.48) compared to women without hypertension. No differences were seen in risk of depression in women with pregnancy-induced hypertension or preeclampsia/eclampsia compared to those without hypertension.
Conclusion
Women with pre-existing hypertension, but not pregnancy induced hypertension are more likely to meet criteria for an antenatal depressive disorder and/or to be treated with antidepressants and could be targeted by obstetricians for screening for depression and enhanced treatment.
Keywords: hypertension, pregnancy, antenatal depression
1. Introduction
Hypertension occurs in up to 13% of all pregnancies [1] and is associated with an increased risk of disseminated intravascular coagulation, abruptio placenta, cerebral hemorrhage, hepatic failure, and acute renal failure [2]. While some women have pre-existing problems with hypertension, elevated blood pressure during pregnancy is estimated to be due to pregnancy related physiologic changes in about 70% of women with hypertension during pregnancy, and returns to normal following delivery.[1] Gestational hypertension is usually defined as hypertension occurring after week 20 of pregnancy, and preeclampsia, which occurs in 5% to 7% of pregnancies, is a severe form of gestational hypertension that is accompanied by proteinuria [2].
Researchers have suggested that serotonin may play a role in the development of pregnancy-related hypertension and preeclampsia because of this neurotransmitter’s potential role in hemostasis and vascular tone in the uteroplacental tissues [3]. Additionally, increased sympathetic nervous system activity may play a role in gestational hypertension and preeclampsia [4]. Depression and anxiety disorders have been linked to both abnormalities in serotonin and sympathetic hyperactivity and depression in early adulthood has been found to be a risk factor for hypertension [5, 6]. Researchers have recently explored links between these psychiatric disorders and gestational hypertension and preeclampsia [7–10].
One large Finnish study of 623 nulliparous women with singleton pregnancies found that after adjusting for confounding factors depression was associated with a 2.5-fold increased risk and anxiety with a 3.2- fold increased risk of preeclampsia [7]. In a case-control study from Peru of 339 women with preeclampsia versus 337 normal pregnant controls, researchers found that those with moderate depression based on the Patient Health Questionnaire-9 (PHQ-9) had a 2.3-fold increased risk of preeclampsia [8]. New research has also suggested that use of SSRIs may be associated with development of preeclampsia [11]. On the other hand a large Dutch study of 3679 women [9] and a large Swedish study of 1495 women[10] did not find evidence that pregnancy-related anxiety or depression was associated with incidence of preeclampsia or pregnancy related hypertension.
Prior studies have not examined whether depression is associated with preexisting hypertension versus pregnancy related hypertension. In this paper, we will examine the association of Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) minor and major depressive disorders and antidepressant use with preexisting hypertension and pregnancy related hypertension (including preeclampsia) in a large sample of women attending a university-based obstetrics clinic.
2. Materials and methods
Participants in this study were patients receiving prenatal care at the University of Washington Obstetrics Clinic between January 2004 and January 2009, who delivered at the University of Washington Hospital. The University’s Obstetrics and Gynecology Clinic and Obstetrics Inpatient Service have linked electronic records. Questionnaires assessing mood and other important psychological and medical information were introduced in January 2004 as a routine part of medical care to all women during pregnancy. All women receiving ongoing obstetrical care and completing at least one questionnaire in either the second or third trimester were eligible for this study. Exclusion criteria included being less than 15 years of age at the time of delivery or inability to complete the questionnaire due to language difficulty or mental incapacity. Once patients filled out a questionnaire, clinic staff was asked to obtain written informed consent to link questionnaire data to automated medical records. All procedures were approved by the University of Washington Human Subjects Institutional Review Board.
2.1. Study variables and measures
The primary independent variable in our study was hypertension in pregnancy (no hypertension, pre-existing hypertension with or without superimposed preeclampsia, and pregnancy induced hypertension including preeclampsia). Diagnosis of pre-existing hypertension was determined by outpatient and inpatient physician ICD-9 diagnoses of 642.0, 642.1, and 642.2 in the automated medical record. Diagnosis of pregnancy induced/gestational hypertension and preeclampsia/eclampsia were based on outpatient and inpatient physician ICD-9 diagnoses of (642.3) and (642.4, 642.5, 642.6 and 642.7) respectively. Those with ICD-9 diagnoses of both pre-existing hypertension and pregnancy-induced hypertension were classified as pre-existing hypertension with superimposed preeclampsia.
Covariate information collected from electronic medical records and questionnaires included demographic characteristics (age, marital status, race/ethnicity, education and employment), general health history, pregnancy history, and social history.
Tobacco status was assessed using the Smoke-Free Families prenatal screen which was developed to screen for smoking in pregnancy [12]. Women with any current smoking were classified as smokers.
Chronic medical problems prior to pregnancy were screened for with a standard list including: hypertension, diabetes, asthma, thyroid disorders, migraines, arthritis, seizure disorders, heart failure, cancer, other heart disease, and chronic physical disability (loss of limb, eyesight, or hearing). Gestational age (weeks) at depression screen was calculated from expected date of delivery and the date the depression screen was administered. A history of pregnancy complications was determined by self-report by women of one or more of the following in a prior pregnancy: gestational diabetes, hypertension or preeclampsia, eclampsia, preterm delivery, preterm labor, placental abruption, preterm rupture of the membranes, hemorrhage, or oligohydramnios. Women with no prior pregnancy were classified as having no history of pregnancy complications.
2.2. Study Outcomes
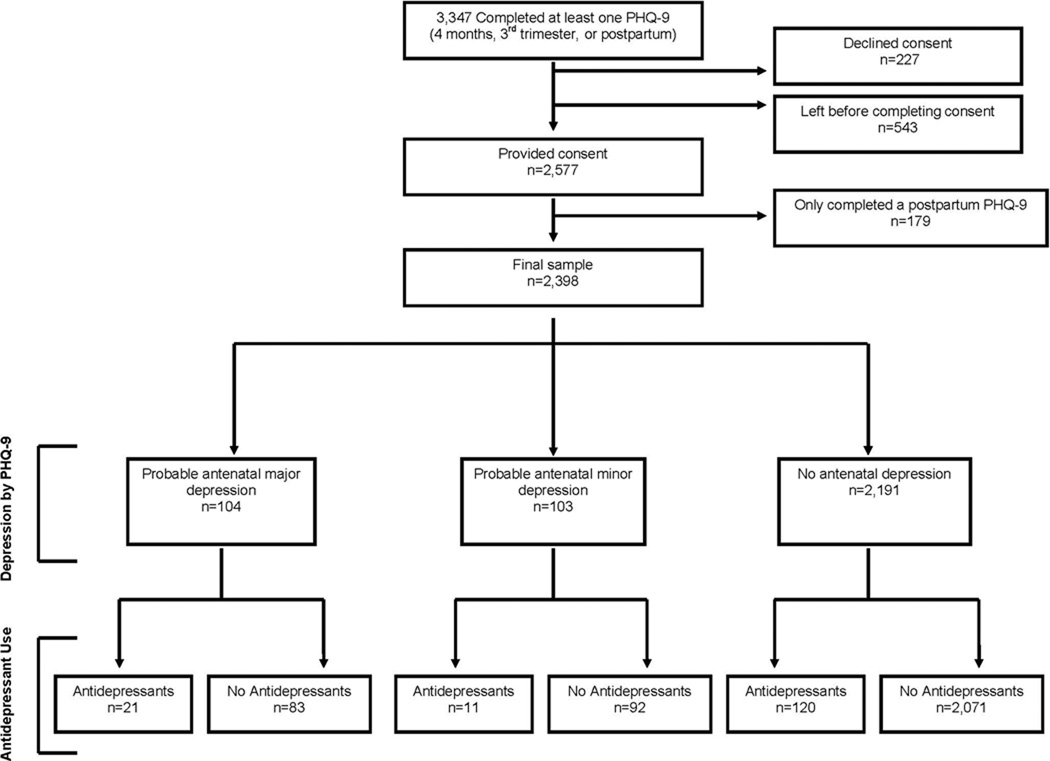
There were two study depression outcomes: Any Depression or Major Depression and/or antidepressant use. Any Depression was defined by a positive diagnosis of probable minor or major depression based on the PHQ-9 [13] or use of any antidepressant medication during the current pregnancy. Major Depression and/or antidepressant use was defined by meeting criteria on the PHQ-9 for probable major depression or any antidepressant use during the current pregnancy [13]. The PHQ-9 diagnosis of major depression is based on DSM–IV criteria and requires the subject to have for at least two weeks five or more depressive symptoms present for more than half the days, with at least one of the symptoms being depressed mood or having anhedonia. The criteria for minor depression requires the subject to have for at least two weeks two to four depressive symptoms for more than half the days, one of which was depressed mood or anhedonia. The PHQ-9 has been validated for a diagnosis of major depression in OB/GYN patients and found to have a sensitivity of 73% and specificity of 98% compared to a structured psychiatric interview diagnosis of major depression [14]. Information about antidepressant use during pregnancy was obtained from the self-report screening questionnaire. Self-report of antidepressant use in pregnancy has high concordance with pharmacy records when collected during pregnancy [15]. Selective serotonin reuptake inhibitors (SSRIs) included: sertraline, fluoxetine, fluvoxamine, paroxetine, citalopram, and escitalopram. All non-SSRI antidepressants were included in the “other antidepressant category”. In the final analysis SSRIs and other antidepressants were combined in the “any antidepressant” category. Figure 1 shows the prevalence of the Any Depression and Major Depression categories and the percent of patients treated with antidepressants.
Fig. 1.
Description of recruitment
2.3. Statistical analyses
The socio-demographic and clinical characteristics of the women in the three hypertension groups were summarized. Chi-square analyses and analyses of variance (ANOVAs) were used to examine group differences. In the event of a significant hypertension group effect, planned post hoc Bonferroni adjusted comparisons were made between each of the 3 pairs of groups to determine the nature of the group differences.
The associations between hypertension and Any Depression (probable minor or major depression or anti-depressant medication use) and hypertension and Major Depression and/or antidepressant use were estimated using logistic regression. Five models were fit sequentially for each depression outcome. The first model included only hypertension status (none, pre-existing hypertension with or without superimposed preeclampsia, pregnancy related hypertension). The second model adjusted for demographic characteristics (maternal age, marital status, non-white race/ethnicity, education, and employment). The third model adjusted for demographic characteristics and the presence of other chronic medical conditions. The fourth model adjusted for demographic characteristics, other chronic medical conditions and current cigarette smoking. The fifth model adjusted for demographics, chronic medical conditions, smoking, and pregnancy variables (prior pregnancy, gestational week at depression screener, and prior pregnancy complications). Odds ratios (OR) and 95% CIs are reported. All analysis were completed using SPSS 18.0 and STATA 10.[16]
2. 4. Missing data and multiple imputation
While data was complete for the primary outcome and exposure there was missing data for most of the covariates. Less than 10% of data was missing for any individual covariate. However, use of complete-case analysis would have resulted in the exclusion of 476 women. Additionally, when data is missing at random use of complete-case analysis biases results and decreases efficiency [17]. Therefore, we used multiple imputation to create 5 complete datasets using ICE [18], in STATA. The regression coefficients from each of the 5 data sets were then combined using the rules described by Little and Rubin [17]. To impute the missing covariate values we specified individual equations using a program, which selects the best imputation equation for each covariate based on the selection rules recommended by van Buuren et. al. [19, 20]. Among the variables considered for each imputation equation were all demographic, pregnancy, and clinical variables in addition to the outcomes of major, minor, any depression, preterm delivery, very preterm delivery, low birth weight, and very low birth weight.
The descriptive tables present the original data (Tables 1 and 2), while the models were all fit using the imputed data sets. In order to clarify the results we ran a sensitivity analysis in which we fit the same series of logistic models using four hypertension groups instead of three: none, pre-existing only, pregnancy related only, and pre-existing with superimposed preeclampsia using multiple imputation methodology. In a second sensitivity analysis, we examined the association of depression with preeclampsia/eclampsia ICD-9 diagnoses by the obstetrician. In a third sensitivity analysis, we examined the association of SSRI use with preeclampsia. In a fourth sensitively analysis, we excluded patients on antidepressants who did not meet criteria for minor or major depression from the definition of depression. In the last sensitivity analysis, we examined results from complete-case analysis (results not shown). The complete-cases analyses results were very similar to those from the multiple imputation.
Table 1.
Study variables stratified by hypertension (N = 2398)
| Total sample N = 2398 M (SD) or N (%) |
No hypertension (HTN) N = 1633 (68.1%) M (SD) or N (%) |
Pre-existing HTN with or without superimposed preeclampsia N = 418 (17.4%) M (SD) or N (%) |
Pregnancy- related HTN only N = 347 (14.5%) M (SD) or N (%) |
Test statistic F(3,2394) or X2(3)- (p) |
|
|---|---|---|---|---|---|
| Maternal age | 30.5 (6.2) | 30.4 (6.1) | 30.8 (6.0) | 30.5 (6.6) | 0.80 (0.45) |
| Non-white race/ethnicity (missing 204, 8.9%) | 762 (34.7%) | 540 (35.8%) | 133 (34.9%) | 89 (29.2%) | 4.92 (0.08) |
| Married or partnered (missing 140, 5.8%) | 1960 (86.8%) | 1342 (87.0%) | 342 (87.0%) | 276 (85.7%) | 0.39 (0.82) |
| Education: some college (missing 142, 5.9%) | 1791 (79.4%) | 1253 (81.2%) | 293 (74.7%) | 245 (76.6%) | 9.66 (0.008) |
| Employment status: full- or part-Time (missing 140, 5.8%) | 1239 (54.9%) | 854 (55.3%) | 203 (51.7%) | 182 (56.5%) | 2.14 (0.34) |
| Number of chronic conditionsa (of 11) (missing 116, 4.8%) | 0.56 (0.90) | 0.51 (0.85) | 0.75 (1.02) | 0.62 (0.94) | 12.65 (< 0.0001) |
| Current tobacco smoking (missing 70, 2.9%) | 174 (7.5%) | 109 (6.8%) | 40 (10.0%) | 25 (7.5%) | 4.47 (0.11) |
| Prior pregnancy | 1799 (75.0%) | 1205 (73.8%) | 347 (83.0%) | 247 (71.2%) | 18.30 (< .0001) |
| gestational week at depression screening | 23.3 (7.3) | 23.5 (7.3) | 22.4 (7.4) | 23.6 (7.4) | 3.83 (0.02) |
| Past pregnancyb complications ≥1 (missing 110) | 519 (22.7%) | 310 (19.8%) | 139 (35.2%) | 70 (21.3%) | 42.94 (< .0001) |
Self reported asthma, arthritis, cancer within 2 years, neurological condition, heart failure, other heart diseases, gastrointestinal. problems, thyroid diseases, migraine headaches or a physical disability – univariate tests show difference due to arthritis, gastrointestinal illness, thyroid and migraine
Includes 6 complications but not gestational diabetes or hypertension or preeclampsia
Table 2.
Mental health and antidepressant use variables stratified by hypertension (N = 2398)
| Total Sample N = 2398 M (SD) or N (%) |
No HTN N = 1633 (68.1%) M (SD) or N (%) |
Pre-existing HTN with or without superimposed preeclampsia N = 418 (17.4%) M (SD) or N (%) |
Pregnancy related HTN only N = 347 (14.5%) M (SD) or N (%) |
Test Statistic F(3,2394) or X2(3) – (p) |
|
|---|---|---|---|---|---|
| PHQ-9 sum | 3.6 (4.0) | 3.4 (3.8) | 4.3 (4.2) | 3.9 (4.3) | 9.22 (< .0001) |
| Probable major depression | 104 (4.3%) | 60 (3.7%) | 24 (5.7%) | 20 (5.8%) | 5.42 (0.07) |
| Probable minor depression | 103 (4.3%) | 63 (3.9%) | 29 (6.9%) | 11 (3.2%) | 9.93 (0.01) |
| Taking SSRI | 124 (5.2%) | 72 (4.4%) | 32 (7.7%) | 20 (5.8%) | 7.44 (0.02) |
| Taking other antidepressantsa | 35 (1.5%) | 20 (1.2%) | 12 (2.9%) | 3 (0.9%) | 7.27 (0.03) |
| Outcome 1: any depression (probable minor or major) or prescribed antidepressants (SSRI or other) | 327 (13.6%) | 196 (12.0%) | 84 (20.1%) | 47 (13.5%) | 18.51 (< .0001) |
| Outcome 2: major depression (probable major depression or prescribed antidepressants (SSRI or other) | 224 (9.8%) | 133 (8.5%) | 55 (14.1%) | 36 (10.7%) | 11.78 (0.003) |
7 patients who were taking both had SSRI and other antidepressant medication
3. Results
During the study period, 3347 women completed at least one psychosocial screening questionnaire at four months gestation, during the third trimester, or postpartum (Figure 1). Staff were present to consent 2577 (77%) for study enrollment. A total of 227 (6.8%) declined to participate in this part of the study and 543 (16.2%) left the clinic before they could be consented. Of the 2577 women who consented, 2398 (93.1%) completed the psychosocial screen at four months gestation or during the third trimester and were eligible for this study. A total of 2071 (86.4%) were in the No Depression group, 327 (13.6%) were in the Any Depression group, and 235 (9.8%) were in the Major Depression group. Although 120 of 154 patients who were taking antidepressants did not meet criteria for minor or major depression, they had a higher mean depression severity score on the PHQ-9 compared to patients who did not meet minor or major depression criteria and were not taking antidepressants ( = = 4.7 ± 3.3 vs.X̄ = 2.7 ± 2.5, t = 6.55 (df = 2189), p < 0.0001)).
The hypertension groups differed in education, number of chronic conditions, number with a prior pregnancy, gestational week at screening and past pregnancy complications (Table 1). Planned post hoc comparisons revealed that the group with pre-existing hypertension compared to those without hypertension during pregnancy had significantly less education (p = 0.005), more chronic medical conditions (p < 0.0001), were more likely to have a prior pregnancy (p < 0.0001), to be screened earlier in pregnancy (p = 0.02) and to have a history of prior pregnancy complications (p < 0.0001). Women with pregnancy-related hypertension compared to those with pre-existing hypertension had fewer prior pregnancies (p < 0.0001) and were less likely to have a history of pregnancy-related complications (p < 0.0001). Women with pregnancy-related hypertension did not differ on any variable compared to those with no hypertension.
Table 2 shows that the three groups had significant differences on all of our depression outcome variables, with the exception of a trend level finding for probable major depression (p = 0.07). Planned post hoc comparisons revealed that these differences were due primarily to greater depression severity or a higher percentage using antidepressants in the pre-existing hypertension group in comparison to those without hypertension (p’s ranged from 0.02 to < 0.0001). Compared to women with pregnancy related hypertension only, women with pre-existing hypertension reported significantly higher prevalence of Any Depression (minor, major or treatment with antidepressants (p = 0.02)) and minor depression (p = 0.02). Women with pregnancy-related hypertension did not differ on any depression variable compared to those with no hypertension.
Table 3 shows the result of the logistic regression analyses. After controlling for sociodemographic variables, the number of chronic medical conditions, smoking and prior pregnancy-related complications, women with pre-existing hypertension compared to pregnant women without hypertension were approximately 55% more likely to meet criteria for Any Depression (probable minor, major or use of antidepressants) and 65% more likely to meet criteria for Major Depression and/or use of antidepressants. Pregnancy-related hypertension was not associated with an increased risk of Any Depression or Major Depression.
Table 3.
Odds ratios (95% CI) for any depression (probable minor or major) or antidepressant use and major depression (probable major depression or antidepressant use) among pregnant women (N = 2398) with pre-existing (N = 418) or pregnancy-related hypertension (N = 347) versus those with no hypertension – using multiple imputation
| Any depression (minor of major) or antidepressant usea N = 327 (13.6%) |
Major depression or antidepressant useb N = 235 (9.8%) |
|||
|---|---|---|---|---|
| Pre-existing HTN | Pregnancy- related HTN |
Pre-existing HTN |
Pregnancy-related HTN | |
| Unadjusted | 1.84h (1.39 – 2.44) | 1.15 (0.82 – 1.62) | 1.82h (1.31 – 2.51) | 1.29 (0.88 – 1.90) |
| Adjusted for | ||||
| Demographicsc | 1.82h (1.36 – 2.44) | 1.12 (0.79 – 1.59) | 1.76h (1.26 – 2.45) | 1.24 (0.84 – 1.84) |
| Demographics +chronic conditionsd | 1.57h (1.15 – 2.13) | 1.04 (0.73 – 1.49) | 1.54f (1.08 – 2.18) | 1.17 (0.78 – 1.74) |
| Demographics + chronic conditionsd + current smoking | 1.55g (1.13 – 2.12) | 1.04 (0.72 – 1.49) | 1.52* (1.07 – 2.16) | 1.17 (0.78 – 1.74) |
| Demographics + chronic conditionsd + current smoking + pregnancy characteristicse | 1.55g (1.08 – 2.23) | 0.92 (0.59 – 1.44) | 1.65f (1.10 – 2.48) | 1.06 (0.65 – 1.73) |
Major depression, minor depression, or antidepressant use vs. no depression
Major depression or antidepressant use vs. no major depression
Demographic characteristics: maternal age at depression screen (years), marital status (married or partnered, single), non-white race/ethnicity, education (some college, high school or less), employment (yes, no)
Number of chronic conditions out of 11
Pregnancy characteristics: prior pregnancy (yes, no), gestational week at depression screen (weeks), ≥1 prior pregnancy complication (yes, no)
p <0.05
p <0.01
p <0.001
The sensitivity analysis that excluded patients currently taking antidepressants who did not meet criteria for minor or major depression from our depression diagnoses found similar odds ratios for the association of major depression with preexisting hypertension (OR = 1.37, 95% CI 0.79 – 2.38) and major and minor depression with preexisting hypertension (OR = 1.44, 95% CI 0.96 – 2.15), but with less certainty in confidence intervals due to lower rates of patients meeting each of these depression criteria.
Table 4 shows the result of the sensitivity analysis in which we separated the pre-existing hypertension group into those with and without superimposed preeclampsia during pregnancy. Women with pre-existing hypertension without superimposed preeclampsia had an approximately 2-fold increased risk of Any Depression (OR = 1.93, 95% CI 1.08, 3.09) and also had a nonsignificant trend toward a higher rate of Major Depression and/or use of antidepressants (OR = 1.62, 95% CI 0.88, 2.98) compared to women without hypertension. Women with pre-existing hypertension with superimposed preeclampsia had a nonsignificant trend toward a higher rate of Any Depression (OR = 1.42, 95% CI 0.93, 2.16) and a significantly higher rate of Major Depression and/or use of antidepressants (OR = 1.66, 95% CI 1.04, 2.65).
Table 4.
Odds ratios (95% CI) for Any Depression (Minor or Major) or Antidepressant Use and Major Depression or Antidepressant Use Among Pregnant Women with Pre-existing Only (N = 131), Pregnancy-Related Only (N=347) and Pre-existing with superimposed preeclampsia (N = 287) Versus Those with No Hypertension (N = 1633) – Using Multiple Imputation
| Any depression (minor of major) or antidepressant usea N = 327 (13.6%) |
Major depression or antidepressant useb N = 235 (9.8%) |
|||||
|---|---|---|---|---|---|---|
| Pre-existing HTN only |
Pregnancy- related HTN |
Both pre-existing & superimposed preeclampsia |
Pre-existing HTN only |
Pregnancy- related HTN |
Both pre-existing & superimposed preeclampsia |
|
| Unadjusted | 2.27h (1.48 – 3.49) | 1.15 (0.82 – 1.62) | 1.66g (1.19 – 2.32) | 2.07g (1.26 – 3.40) | 1.29 (0.88 – 1.90) | 1.70** (1.16 – 2.49) |
| Adjusted For | ||||||
| Demographicsb | 2.29h (1.47 – 3.57) | 1.12 (0.79 – 1.59) | 1.62g (1.15 – 2.29) | 2.00g (1.20 – 3.33) | 1.24 (0.84 – 1.84) | 1.64* (1.11 – 2.43) |
| Demographics +chronic conditionsd | 1.98h (1.25 – 3.13) | 1.04 (0.73 – 1.49) | 1.39 (0.97 – 2.00) | 1.76f (1.04 – 2.98) | 1.18 (0.80 – 1.76) | 1.43 (0.95 – 2.16) |
| Demographics + chronic conditionsd + current smoking | 1.94g (1.22 – 3.08) | 1.04 (0.72 – 1.49) | 1.38 (0.96 – 1.99) | 1.71g (1.01 – 2.91) | 1.18 (0.79 – 1.76) | 1.43 (0.95 – 2.16) |
| Demographics + chronic conditionsd + current smoking + pregnancy characteristicse | 1.93f (1.08 – 3.09) | 0.92 (0.59 – 1.43) | 1.42 (0.93 – 2.16) | 1.62 (0.88 – 2.98) | 1.06 (0.65 – 1.73) | 1.66* (1.04 – 2.65) |
Major depression, minor depression, or antidepressant use vs. no depression
Major depression or antidepressant use vs. no major depression
Demographic characteristics: maternal age at depression screen (years), marital status (married or partnered, single), non-white race/ethnicity, education (some college, high school or less), employment (yes, no)
Number of chronic conditions out of 10
Pregnancy characteristics: prior pregnancy (yes, no), gestational week at depression screen (weeks), ≥1 prior pregnancy complication (yes, no)
p <0.05
p <0.01
p <0.001
Our next sensitivity analyses found no association with either Any Depression or Major Depression and/or use of antidepressants with preeclampsia/eclampsia ICD-9 diagnoses (OR = 0.87, 95% CI 0.61, 1.25) and (OR = 0.94, 95% CI 0.62, 1.41), respectively) in fully adjusted models. A last sensitivity analysis found no association between SSRI use and preeclampsia (OR = 1.09, 95% CI 0.66, 1.82).
4. Discussion
In this study, pregnant women with pre-existing hypertension with or without superimposed preeclampsia during pregnancy were 55% to 65% more likely to meet criteria for significant depressive symptoms or to be taking antidepressants. Compared to women without hypertension, women with pre-existing hypertension without superimposed preeclampsia were approximately twice as likely and those with pre-existing hypertension with superimposed preeclampsia were 42% to 66% more likely to meet criteria for depression or to be taking antidepressant medication. Women with ICD-9 diagnoses of preeclampsia or eclampsia had similar rates for meeting criteria for depression or to be using antidepressants as those without hypertension. SSRI use alone was not associated with preeclampsia.
Women with pre-existing hypertension are an important group for obstetricians to screen for depression since depression in pregnancy has been associated with a higher risk of preterm birth and low birth weight as well as postpartum depression [21]. Depression in patients with chronic medical illnesses is associated with poor adherence to medical regimens such as following diet and exercise regimens and taking medications as prescribed. 22]. Approximately 10% to 25% of women with pre-existing hypertension go on to develop superimposed preeclampsia[2] and understanding high risk groups is essential to target treatments. Future studies need to examine whether depression in pregnant women affects adherence to anti-hypertension medication as well as progression in women with pre-existing hypertension to worsening hypertension and preeclampsia.
No relationship was found between depression and pregnancy related hypertension, including preeclampsia. This finding is consistent with two of the largest published studies [9, 10], but differs from some earlier epidemiologic studies that suggested depression was associated with preeclampsia [7, 8]. These prior studies didn’t differentiate whether the diagnosis of preeclampsia was superimposed on pre-existing hypertension or was only due to pregnancy related hypertension.
This study assessed prevalent depression during pregnancy, therefore, depression may have been present prior to pregnancy and may have contributed to the development of pre-existing hypertension. Depression may increase risk of hypertension prior to pregnancy because of both health risk behaviors and the psychobiologic changes associated with this disorder. Depression in females in teenage to early adult years has been found to be a risk factor for development of obesity [5, 6]. Obesity is linked to development of metabolic syndrome, including hypertension. Depression may also be linked to development of hypertension because of increases in sympathetic nervous system tone [23]. Depression’s effect on the autonomic nervous system has been posited as causing the following cardiovascular risk factors found in patients with depression: decreased heart rate variability [24], increased resting and 24 hour heart rates, increased heart rate to orthostatic challenge [25] and increased QT interval variability [26], reflecting abnormal ventricular repolarization.
Limitations include that the study was carried out in one large obstetrics clinic in one geographic region of the United States, lack of structured psychiatric interviews to confirm PHQ-9 diagnosis and history of prior depressive episodes, lack of history of childhood adversity or domestic violence, and cross-sectional data which limited assessment of causal relationships. We also did not assess BMI, but did control for type 2 diabetes, which is associated with high BMI.
Strengths of the study include the large sample size, use of a routine screening protocol with the high rates of response, the use of automated data and physician outpatient clinic and inpatient diagnoses rather than self-report diagnosis, and the use of DSM-IV diagnostic criteria for depression.
5. Conclusion
In conclusion, pregnant women with pre-existing hypertension with or without superimposed preeclampsia were found to have a higher risk of depression. Given the potential adverse effect of depression on birth outcomes as well as adherence to hypertension treatment, this is an important group of women that Ob-Gyn physicians should target for depression screening.
Acknowledgements
This work was supported by K24 MH069741 (PI: W. Katon) from the National Institute of Mental Health. J. Katon was supported by a Reproductive, Perinatal and Pediatric Training Grant (T32 HD052462) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the Samuel and Althea Stroum Fellowship, and a grant from the Seattle chapter of Achievement Rewards for College Scientists.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
Wayne Katon is on the speaker’s bureau for Forest, Wyeth, Eli Lilly and Pfizer pharmaceutical companies and is an advisory committee member for Eli Lilly and Wyeth.
References
- 1.National High Blood Pressure Education Program Working Group Report on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 1990;163:1691–1712. doi: 10.1016/0002-9378(90)90653-o. [DOI] [PubMed] [Google Scholar]
- 2.Jim B, Sharma S, Kebede T, Acharya A. Hypertension in pregnancy:a comprehensive update. Cardiol Rev. 2010;18:178–189. doi: 10.1097/CRD.0b013e3181c60ca6. [DOI] [PubMed] [Google Scholar]
- 3.Bolte A, van Geijn H, Dekker G. Pathophysiology of preeclampsia and the role of serotonin. Eur J Obstet Gynecol Reprod Biol. 2001;95:12–21. doi: 10.1016/s0301-2115(00)00367-5. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood JP, Scott EM, Walker JJ, Stoker JB, Mary DA. The magnitude of sympathetic hyperactivity in pregnancy-induced hypertension and preeclampsia. Am J Hypertens. 2003;16:194–199. doi: 10.1016/s0895-7061(02)03256-9. [DOI] [PubMed] [Google Scholar]
- 5.Richardson LP, Davis R, Poulton R, McCauley E, Moffitt TE, Caspi A, et al. A longitudinal evaluation of adolescent depression and adult obesity. Arch Pediatr Adolesc Med. 2003;157:739–745. doi: 10.1001/archpedi.157.8.739. [DOI] [PubMed] [Google Scholar]
- 6.Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics. 2002;110:497–504. doi: 10.1542/peds.110.3.497. [DOI] [PubMed] [Google Scholar]
- 7.Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95:487–490. doi: 10.1016/s0029-7844(99)00602-x. [DOI] [PubMed] [Google Scholar]
- 8.Qiu C, Sanchez S, Lam N, Garcia P, Williams M. Associations of depression and depressive symptoms with preeclampsia: results from a Peruvian case-control study. BMC Womens Health. 2007;27:7–15. doi: 10.1186/1472-6874-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollebregt K, van der Wal M, Wolf H, Vrijkotte T, Boer K, Bonsel G. Is psychosocial stress in first ongoing pregnancies associated with pre-eclampsia and gestational hypertension? BJOG. 2008;115:607–615. doi: 10.1111/j.1471-0528.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- 10.Andersson L, Sundström-Poromaa I, Wulff M, Aström M, Bixo M. Implications of antenatal depression and anxiety for obstetric outcome. Obstet Gynecol. 2004;104:467–476. doi: 10.1097/01.AOG.0000135277.04565.e9. [DOI] [PubMed] [Google Scholar]
- 11.Toh S, Mitchell A, Louik C, Werler M, Chambers C, Hernández-Díaz S. Selective serotonin reuptake inhibitor use and risk of gestational hypertension. Am J Psychiatry. 2009;166:320–328. doi: 10.1176/appi.ajp.2008.08060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melvin CL, Tucker P. Measurement and definition for smoking cessation intervention research: the smoke-free families experience. Smoke-Free Families Common Evaluation Measures for Pregnancy and Smoking Cessation Projects Working Group. Tob Control. 2000;9 Suppl 3:III87–III90. doi: 10.1136/tc.9.suppl_3.iii87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer RL, Williams JB, Kroenke K, Hornyak R, McMurray J. Validity and utility of the PRIME-MD patient health questionnaire in assessment of 3000 obstetric-gynecologic patients: the PRIME-MD Patient Health Questionnaire Obstetrics-Gynecology Study. Am J Obstet Gynecol. 2000;183:759–769. doi: 10.1067/mob.2000.106580. [DOI] [PubMed] [Google Scholar]
- 15.Newport DJ, Brennan PA, Green P, Ilardi D, Whitfield TH, Morris N, et al. Maternal depression and medication exposure during pregnancy: comparison of maternal retrospective recall to prospective documentation. Bjog. 2008;115:681–688. doi: 10.1111/j.1471-0528.2008.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stata Statistical Software. College Station, TX: Statacorp LP; 2005. [Google Scholar]
- 17.Little R, Rubin D. Statistical Analysis with Missing Data. New York, NY: John Wiley &Sons, Inc.; 2002. [Google Scholar]
- 18.Royston P. Multiple imputation of missing valudes: update of ICE. Stata Journal. 2005;B:527–536. [Google Scholar]
- 19.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 20.Medieiros R. 'pred_eq' A program that creates and tests imputation models (equations) for ICE. UCLA Academic Technology Service. 2010 [Google Scholar]
- 21.Grote N, Bridge J, Gavin A, Melville J, Iyengar S, Katon W. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin EH, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 23.Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121:S20–S27. doi: 10.1016/j.amjmed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Carney RM, Rich MW, teVelde A, Saini J, Clark K, Freedland KE. The relationship between heart rate, heart rate variability and depression in patients with coronary artery disease. J Psychosom Res. 1988;32:159–164. doi: 10.1016/0022-3999(88)90050-5. [DOI] [PubMed] [Google Scholar]
- 25.Carney RM, Freedland KE, Veith RC, Cryer PE, Skala JA, Lynch T, et al. Major depression, heart rate, and plasma norepinephrine in patients with coronary heart disease. Biol Psychiatry. 1999;45:458–463. doi: 10.1016/s0006-3223(98)00049-3. [DOI] [PubMed] [Google Scholar]
- 26.Carney RM, Freedland KE, Stein PK, Watkins LL, Catellier D, Jaffe AS, et al. Effects of depression on QT interval variability after myocardial infarction. Psychosom Med. 2003;65:177–180. doi: 10.1097/01.psy.0000033129.21715.4b. [DOI] [PubMed] [Google Scholar]