Table 2.
Stereospecific cross coupling of ethyl-Z-3-iodoacrylate with 3,5-dimethoxyphenol
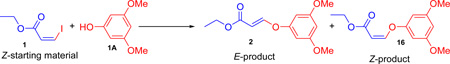 | |||||||
|---|---|---|---|---|---|---|---|
| entry | catalyst | eq. base | solvent | temp. (°C) | time (h) | yield (%)a | Z/E |
| 1 | CuI, L | 2.0 Cs2CO3 | DMF | 40 | 0.5 | 95 | 0:100 |
| 2 | CuI, L | 2.0 Cs2CO3 | DMF | rt | 0.5 | 94 | 40:60 |
| 3 | CuI | 2.0 Cs2CO3 | DMF | rt | 0.5 | 95 | 40:60 |
| 4 | L | 2.0 Cs2CO3 | DMF | rt | 0.5 | 95 | 40:60 |
| 5 | - | 2.0 Cs2CO3 | DMF | 40 | 0.5 | 94 | 0:100 |
| 6 | - | 2.0 Cs2CO3 | DMF | rt | 0.5 | 95 | 40:60 |
| 7 | - | 2.0 Cs2CO3 | toluene | rt | 6 | 88 | 70:30 |
| 8 | - | 2.0; K2CO3 | DMF | rt | 0.5 | 91 | 40:60 |
| 9 | - | 2.0; K2CO3 | toluene | rt | 6 | 75 | 65:35 |
| 10 | - | 2.0; K3PO4 | DMF | rt | 0.5 | 93 | 40:60 |
| 11 | - | 2.0; K3PO4 | toluene | rt | 10 | 60 | 65:35 |
| 12 | - | 2.0; HMDS | DMF | rt | 6 | 10 | 100:0 |
| 13 | - | 2.0; HMDS | toluene | rt | 15 | 10 | 100:0 |
| 14 | - | 2.0; LiHMDS | DMF | rt | 6 | 0 | N/A |
| 15 | - | 2.0 DABCO | DMF | rt | 1.0 | 95 | 100:0 |
| 16 | - | 1.1 DABCO | DMF | rt | 6.0 | 84 | 100:0 |
| 17 | - | 0.5 DABCO | DMF | rt | 2.5 | 50 | 100:0 |
| 18 | - | 0.2 DABCO | DMF | rt | 2.5 | 20 | 100:0 |
| 19 | - | 0.1 DABCO | DMF | rt | 2.5 | 10 | 100:0 |
| 20 | CuI, L | 2.0 DABCO | DMF | rt | 0.5 | 95 | 100:0 |
| 21 | CuI, L | 2.0 DABCO | toluene | rt | 12 | 87 | 100:0 |
| 22 | CuI | 2.0 DABCO | DMF | rt | 0.5 | 95 | 100:0 |
| 23 | CuI | 2.0 DABCO | toluene | rt | 12 | 84 | 100:0 |
| 24 | - | 2.0 DMAP | DMF | rt | 6 | 0 | N/A |
| 25 | CuI, L | 2.0 DMAP | DMF | rt | 6 | 0 | N/A |
| 26 | - | 2.0 Et3N | DMF | rt | 6 | 0 | N/A |
| 27 | CuI, L | 2.0 Et3N | DMF | rt | 6 | 0 | N/A |
| 28 | - | 2.0 DBU | DMF | rt | 6 | 0 | N/A |
| 29 | CuI, L | 2.0 DBU | DMF | rt | 6 | 0 | N/A |
The starting aryl vinyl halides contained ~ 3–9 % Z-isomer; this resulted in ~3–9 % of the cis-isomer, included in the overall yield.
