Abstract
Sepharose ion-exchange particles bearing strong Lewis acid/base functional groups (sulfopropyl, carboxymethyl, quarternary ammonium, dimethyl aminoethyl, and iminodiacetic acid) exhibiting high plasma protein adsorbent capacities are shown to be more efficient activators of blood factor XII in neat-buffer solution than either hydrophilic clean-glass particles or hydrophobic octyl sepharose particles ( ; a.k.a autoactivation, where FXII is the zymogen and FXIIa is a procoagulant protease). In sharp contrast to the clean-glass standard of comparison, ion-exchange activators are shown to be inefficient activators of blood plasma coagulation. These contrasting activation properties are proposed to be due to the moderating effect of plasma-protein adsorption on plasma coagulation. Efficient adsorption of blood plasma proteins unrelated to the coagulation cascade impedes FXII contacts with ion-exchange particles immersed in plasma, reducing autoactivation, and causing sluggish plasma coagulation. By contrast, plasma proteins do not adsorb to hydrophilic clean glass and efficient autoactivation leads directly to efficient activation of plasma coagulation. It is also shown that competitive-protein adsorption can displace FXIIa adsorbed to the surface of ion-exchange resins. As a consequence of highly-efficient autoactivation and FXIIa displacement by plasma proteins, ion-exchange particles are slightly more efficient activators of plasma coagulation than hydrophobic octyl sepharose particles that do not bear strong Lewis acid/base surface functionalities but to which plasma proteins adsorb efficiently. Plasma proteins thus play a dual role in moderating contact activation of the plasma coagulation cascade. The principal role is impeding FXII contact with activating surfaces but this same effect can displace FXIIa from an activating surface into solution where the protease can potentiate subsequent steps of the plasma coagulation cascade.
Keywords: Autoactivation, FXII, plasma coagulation, ion-exchange
1. Introduction
Materials exhibiting ion-exchange properties, the exchange of an ion in solution for a surface-bound ion of the same charge, rely on surface functional groups that exhibit strong Lewis acid/base properties. Surface functional groups on ordinary polymers, such as carbonyl or ether for examples, are relatively weak Lewis bases that are capable of hydrogen bonding to water but typically do not exhibit ion-exchange properties, especially in high-ionic strength media such as phosphate-buffered saline (PBS) wherein surface charges are highly screened. By contrast, surface functional groups such as sulfopropyl (-CH2-SO3−, SP), carboxymethyl (-CH2-COOH, CM), quarternary ammonium (NR4+, Q), dimethyl aminoethyl ((CH3)2-N-(CH2-CH2)-, DEAE) of particular interest herein (see Table 1) are sufficiently strong Lewis bases (first two) or acids (last two) that a strong electric field surrounds these moieties even in PBS, conferring unusual and useful properties such as ion-exchange (anion and cation-exchangers, respectively). Iminodiacetic acid (HN(CH2CO2H)2, CH) included in this study is a metal chelator with strong Lewis basicity.
Table 1.
Surface Activation of Plasma Coagulation
| Type | Activator Name (Acronym) |
Ion exchange capacity (Ave., mmol/mL) |
Advancing Contact Angle (degrees) |
τ0 (mL/m2) |
Kact (mL/m2) |
R2 (%) |
|---|---|---|---|---|---|---|
| Sepaharose-Based Resins |
Sulfopropyl (SP) | −0.18-0.25 | - | - | (1.6±0.2)×10−2 (5±3)×10−2 |
83.0 78.6 |
| Carboxy methyl (CM) | −0.09-0.13 | - | - | (4±2)×10−3 ~10−4 |
73.4 - |
|
| Quaternary ammonium (Q) | 0.18-0.25 | - | - | ~10−4 | - | |
| Diethyl aminoethyl (DEAE) | 0.11-0.16 | - | - | (7±1)×10−3 | 92.8 | |
| Iminodiacetic Acid Chelating (CH) | 0.30-0.37 | - | - | ~10−5 | - | |
| Octadecyl Sepharose (OS) | - | - | - | (5±1)×10−2 ~10−4 |
64.5 - |
|
| Treated Glass Particles |
Nyebar | - | 110.5 ± 2.8 | -25.2 ± 3.1 | (9±2)×10−3 | 86.1 |
| OTS | - | 102.3 ± 2.6 | −15.3 ± 3.1 | 0.13±0.02 | 97.0 | |
| PTES | - | 93.4 ± 1.5 | −4.3 ± 1.9 | (5±1)×10−2 | 97.2 | |
| VTES | - | 85.1 ± 1.7 | 6.1 ± 2.1 | 0.19±0.04 | 93.4 | |
| APTES | - | 54.6±0.4 | 41.7±2.6 | 10−2 | - | |
| Clean Glass | - | 0 | 72 | 19.2±1.9 | 99.4 |
Notes: Silane treated glass particles: Nyebar = 1,1- pentadecaflurooctylmethacrylate deposited on OTS-treated particles; OTS = octadecyltricholorosilane; PTES = n-propyltriethoxysilane; VTES = vinyltriethoxysilane; APTES = aminopropyltriethoxysilane; Clean glass = oxidized glass particles. Error in Θ is standard deviation of the mean of N = 3 measurements. Advancing water adhesion tension in dyne/cm, where mJ/m2 is buffer interfacial tension and Θ is the advancing contact angle. Error in τo calculated by propagation of error in replicate contact angle readings. Kact is a measure of procoagulant catalytic potential derived by fitting surface-area titration data to a model with R2 goodness of fit to that model. Values listed without R2 are best estimates. Two Kact values correspond to plasma lot #1 and #2, single value refers to plasma lot #1.
A special property of ion-exchange surfaces of potential utility in biomedical/biotechnical applications is the unusually high adsorbent capacity for blood proteins compared even to hydrophobic adsorbents that typically exhibit the highest adsorbent capacities among ordinary materials [1]. This is to be contrasted with low (or zero) adsorbent capacity of hydrophilic surfaces bearing weaker Lewis acid/base functionalities of the type mentioned above [2-5]. Proteins adsorb to ion-exchange functionalities through an ion-exchange mechanism, even from PBS solution, wherein the interaction of protein with the electric field surrounding the acid/base group is sufficiently strong that protein can displace surface-adsorbed water and enter the adsorbed state [1]. By contrast, energetics of protein interaction with hydrophilic surfaces bearing weaker Lewis acid/base functionalities such as those on clean glass or oxidized polymers are insufficient to displace surface-adsorbed water [1-4, 6-13]. Effectively, protein adsorption to hydrophilic surfaces is blocked by surface-adsorbed water until Lewis acid/base strength of surface functional groups rises to that of the ion-exchange category. At that point, protein adsorption by an ion-exchange-like process becomes possible.
Another property of ion-exchange functionalities of interest to biomaterials is that quarternary ammonium (Lewis acid, cationic) surfaces do not seem to activate the plasma coagulation cascade [14, 15], even though these materials can exhibit hydrophilicity comparable to surfaces bearing Lewis base (anionic) functionalities typically associated with strong activation of the plasma coagulation cascade (e.g. glass, oxidized polymers, kaolin; see ref. [16] which is a review of contact activation literature to 2008 and citations therein). The exact reasons for this are not clear, but then the biophysics underlying contact activation of blood plasma coagulation is an open issue in biomaterials surface science [16].
Our investigations into contact activation of plasma coagulation have revealed that the first step of the coagulation cascade, activation of the zymogen FXII into the protease form FXIIa, is moderated by the protein composition of the fluid phase in which FXII is dissolved [17, 18]. This surface-catalyzed reaction is sometimes referred to as autoactivation () and may produce other proteases such as FXIIf that also exhibit procoagulant properties [16, 17]. Autoactivation of FXII in pure buffer solution generates a parabolic yield of procoagulant enzymes (putatively FXIIa) as a function of activator hydrophilicity, high at both hydrophobic and hydrophilic extremes of the wetting continuum but falling through a broad minimum between these extremes. In plasma or high-concentration protein cocktails, however, autoactivation by hydrophobic activators is very much lower than by hydrophilic counterparts. The proposed explanation of these experimental observations is that wholesale adsorption of blood proteins unrelated to plasma coagulation (e.g. albumin, IgG, transferrin, etc.) to hydrophobic activators from plasma or protein cocktails impedes surface-FXII interactions, causing low autoactivation yield and consequently sluggish coagulation – a phenomenon we have termed an “adsorption-dilution effect” [16]. By contrast, blood proteins do not adsorb to hydrophilic activators and no such adsorption-dilution effect can occur. Instead, random “collisions” of FXII with hydrophilic surfaces immersed in plasma are apparently sufficient to induce autoactivation and cause rapid plasma coagulation. Hence, the traditionally-observed specificity of plasma coagulation for hydrophilic-anionic activators is really only an apparent specificity induced by adsorption competition between FXII and a plethora of other plasma proteins at hydrophobic surfaces that does not occur at hydrophilic surfaces [16].
Discovery of the adsorption-dilution effect prompts us to hypothesize that inefficient activation of blood plasma coagulation by hydrophilic-cationic surfaces is due to the adsorption-dilution effect occurring at protein-adsorbent ion-exchange-type surfaces in a way that parallels operation of the adsorption-dilution effect at hydrophobic-activator surfaces. That is to say, we propose a single mechanism to explain how it happens that both hydrophobic and hydrophilic-cationic surfaces are inefficient activators of plasma coagulation whereas hydrophilic-anionic surfaces are efficient contact activators of plasma coagulation. Herein we test this hypothesis by comparing plasma coagulation activation and FXII autoactivation in neat PBS solutions using commercial sepharose-based ion-exchange resins bearing either strong Lewis acid or base surface functionalities as model activators. Hydrophobic octyl sepharose with no ion-exchange capacity, hydrophilic clean glass, and silanized glass particles incrementally sampling the observable water-wetttability range are used as a standard of comparisons. Observations are interpreted in terms of previous work measuring protein adsorption to these ion-exchange resins in comparison to octyl sepharose [1].
2. Methods and Materials
2.1 Sepharose-based Activator Particles
Octyl Sepharose™ 4 Fast Flow adsorbent (S), Q Sepharose™ Fast Flow adsorbent, DEAE Sepharose™ Fast Flow adsorbent (DEAE) , SP Sepharose™ Fast Flow adsorbent (SP), CM Sepharose™ Fast Flow adsorbent (CM), and iminodiacetic acid Chelating Sepharose™ Fast Flow (CH) resins were obtained from GE Healthcare (Table 1). Ion-exchange capacities provided by the vendor were accepted without further analysis and octyl sepharose was assigned a null ion-exchange capacity in recognition of the fact that this hydrophobic chromatographic material bears no polar functional groups [1]. These particles were used as model activators of both blood factor XII and plasma coagulation with different Lewis acid/base strength, as described further below. Ion-exchange activator particles listed in Table 1 were identical in all respects except for surface chemistry [19]. The vendor specified 40% by volume particles dispersed in 20% aqueous ethanol solution with a particle size distribution of 45 -165 μm and 90 μm nominal diameter. These sepharose-based activators were freshly prepared just before use in activation experiments by 3X washing in PBS (to remove ethanol) using a sequential centrifugation/resuspension protocol (6000 RPM for 2 min in a Hettick microtube fixed-rotor centrifuge, VWR) that processed 1 mL of as-received suspension (600 μL fluid, 400 μL particles). After each of 3 centrifugations, 500 μL of supernatant was replaced with 500 μL PBS, ending with a 60:40 v/v stock suspension in PBS. Particles were re-suspended by gentle pipette aspiration.
Geometric surface area of dispensed particles was based on nominal bead diameter specified by the vendor. The total volume of dispensed particles was based on the volume % of the particle solution and the aliquot volume. The total number of dispensed particles was calculated from the nominal volume of a single bead. The total dispensed surface area was calculated from the nominal area per bead. It was assumed that the precision of dispensed surface area was limited by precision of volumetrics using ordinary laboratory pipettes. The actual surface area of these hydrogel particles was not measured because accurate knowledge of activator surface area was not necessary in this comparative study (see further below) and because of insuperable analytical difficulties encountered in working with relatively low-nominal-surface-area, hydrated-hydrogel particles that precluded surface-area determinations at a size scale relevant to proteins.
Silanized glass-particle activators listed in Table 1 were 425-600 μm diameter glass particles (Sigma Aldrich) in either cleaned or silanized form. The nominal specific area used in this work was 5×10−3 m2/g (based on 512.5 μm mean diameter and 168 μg/particle). Silanes (used as received from Sigma Aldrich) applied in this work were octadecyltricholorosilane (OTS), 3-aminopropyltriethoxysilane (APTES), n-propyltriethoxysilane (PTES), and vinyltriethoxysilane (VTES). OTS-treated glass particles were optionally coated in a 0.2% solution of 1,1- pentadecaflurooctylmethacrylate in tricholorotrifloroethane (“Nyebar”, Nye Lubricants, Fairhaven, MA) by immersion followed by air drying. Glass cover slips (Fisher 22 × 30 × 0.1mm) were subjected to all surface treatments for particles as described below, providing a substrate suitable for reading phosphate buffer saline (PBS, Sigma) contact angles reported in column 4 of Table 1 and converted to water adhesion tension ; where is pure buffer interfacial tension 71.97 mJ/m2 and Θa is the advancing contact angle at 20 °C.
Glass particles and cover slips were first cleaned and activated by 30 min. immersion in heated piranha solution (30% H2O2 in concentrated H2SO4 at approximately 80 °C) followed by 3X sequential washes in each of 18 MΩ de-ionized water and ethanol. Piranha-solution oxidized glass was air dried and subsequently oxidized by air-plasma treatment (10 min at 100 W plasma; Herrick, Whippany, NY) of a single layer of particles (or cover slip) held in a 15 mm Pyrex glass Petri dish, directly before use in silanization procedures or activation measurements. Glass surfaces treated in this manner were found to be fully water wettable and designated “clean glass”. Clean glass particles and cover slip samples were silanized by 1.5 hr reaction with 5% v/v OTS in chloroform. Silanized samples were 3X rinsed with chloroform before curing in a vacuum oven at 110 °C for 12 hr. Cured OTS samples were optionally immersed in Nyebar solution for 10 min and air dried to produce a surface slightly more hydrophobic than rendered by OTS treatment alone (see Table 1). APTES silanization was carried out by 20 min reaction of clean glass with 95:5 v/v ethanol/water solution with 5% APTES that had been hydrolyzed overnight in the ethanol-water mixture before use. APTES-treated glass was 3X washed with ethanol and cured overnight in a vacuum oven at 110 °C. Silanization with PTES and VTES followed the APTES procedure, except that 90:10 v/v ethanol-water solution containing 0.5 % glacial acetic acid was used.
We assumed that the nominal surface area of ion-exchange particles could be compared to the nominal surface area of glass particles with no correction in full recognition of the fact that unmeasured differences in dispensed surface area would introduce systematic error in quantitative comparison of activation properties on an absolute basis. However, only qualitative comparisons of these two activator types were made in this work and unmeasured differences in surface area were therefore not considered critical to the interpretation of experimental results. Quantitative comparison of activation properties as a function of surface area within either ion-exchange or glass-particle types was not affected by surface area estimates since each activator type was prepared by weight (glass) or volume (ion exchange) from sources with fixed specific surface area.
Ion-exchange resins exhibited a high protein-adsorption capacity compared to hydrophobic OS [1]. Protein adsorption capacity of silanized-glass particles decreased with increasing hydrophilicity to zero near a nominal water contact angle Θ = 65° (see, for examples, refs. [2, 3, 5, 20, 21] and citations therein). Measurement of the FXIIa solution yield (Section 2.5) was therefore affected by activator surface chemistry to an unknown extent because an unmeasured quantity of FXIIa possibly remained adsorbed to the surface, depending on activator surface chemistry. In light of the above protein-adsorption trends, we assumed that measurement of FXII activation by ion-exchange, OS, and OTS-glass activators were underestimates compared to that of clean glass which did not adsorb protein. This underestimation was not explicitly included in the interpretation of results and did not alter basic conclusions because correction for this underestimation would only emphasize the contrast between high FXII activation in buffer and low plasma coagulation activation (see further Sections 2.6, 3.4, and 4.3).
2.2 Proteins and Plasma
Two lots of human FXII were used as received from Haematologic Technologies Inc. (Essex Junction, VT) and one lot of FXIIa was used as received from Enzyme Research Laboratories (South Bend, IN). Coagulation time of re-calcified platelet poor plasma (see below) spiked with 30 μg/mL FXII (nominal physiological concentration) was comparable to blank (coagulation time of un-supplemented citrated plasma, see Section 2.4), demonstrating that FXII was not contaminated with measurable quantities of FXIIa. Activity of both FXII and FXIIa specified by the vendor was accepted without further confirmation. FXIIa activity was specified in the traditional units of plasma-equivalent-units-per-mL (PEU/mL) [22]. FXIIa concentrations in PEU/mL were converted to mg/mL for activation yield calculations using the vendor-supplied conversion factor of 76.4 PEU/mg for human FXII (see further Appendix A of ref. [17]). Neat-buffer solutions of FXII and FXIIa solutions were prepared in phosphate buffer saline (PBS; Sigma; 0.14M NaCl, 3mM KCL prepared from powder in 18MΩ de-ionized water at pH = 7.2).
Citrated (ACD) human platelet-poor plasma (PPP) was prepared from outdated (within 2 days of expiration) lots obtained from the M.S. Hershey Medical Center Blood Bank. This work was performed with a two different lots (designated I and II) of pooled plasma aliquoted into 15 ml polypropylene tubes (Falcon, Becton Dickinson) and frozen at −20 °C until use. Relatively minor quantitative variations arose within these plasma lots over about one year of experimentation, possibly due to changes in coagulation factor activity during storage [23-25]. Experience has also shown that different lots of plasma yield quantitatively different but qualitatively similar results. Correction for differences between plasma lots I and II was made by measuring FXIIa-titration calibration curves for each lot (see below). Coagulation time by contact with clean glass activators was monitored throughout this study as a plasma quality-assurance measure. Plasma was discarded when this coagulation time differed from that measured at time of initial preparation by more than 5 minutes.
2.3 FXIIa Assay
Plasma coagulation time (CT) was used as the traditional hematology method to quantify FXIIa by appealing to an “FXIIa titration” calibration curve that related CT to FXIIa concentration (expressed either in PEU/mL or mg/mL) [22, 26]. Protocol for the FXIIa assay applied in this work has been described in detail elsewhere [15, 18, 20, 27, 28]. Briefly, FXIIa titrations were carried out by equilibrating 500 μL of thawed PPP in 12 × 75 mm polystyrene tubes (VWR) to ambient temperature, mixing with increasing volumes/concentrations of FXIIa solution in PBS, and diluting with sufficient additional PBS to bring total volume to 900 μL. Coagulation was induced by recalcification with 100 μL of 0.1 M CaCl2 and tube contents were mixed on a slowly-turning hematology mixer (Roto-shake Genie, Scientific Industries, Inc.). CT after recalcification was noted by a distinct change in fluid-like rheology to gel formation, allowing determination of a coagulation endpoint to within 10 sec. or so [15, 20, 29]. So-measured CT was observed to be exquisitely sensitive to FXIIa with a minimum quantifiable concentration of ~ 9.7 ×10−4 PEU/mL. FXIIa titration curves in PPP were linear over the concentration range of interest to this work when scaled on a logarithmic concentration axis. Calibration curves were fit to y = m log10 x + c by linear least squares regression; where x is FXIIa concentration in PEU/mL, m and c were adjustable parameters of the fit (m= −7.98 ± 0.33, c = 8.66 ± 0.53, R2 = 95.0% from triplicate determinations using plasma lot I; m=−5.15 ± 0.19, c = 4.81 ± 0.32, R2 = 95. from triplicate determinations using plasma lot II).
Measurement of CT induced by addition of solutions containing unknown concentrations of FXIIa was used in combination with calibration curves to ascertain FXIIa produced in activation experiments described below. Strictly speaking, this FXIIa assay measured net plasma-coagulation-inducing activity of the product(s) resulting from contact activation of FXII in buffer solution, reported herein in terms of apparent FXIIa activity computed from the calibration curve. As such, the coagulation assay did not discriminate among various activated fragments of FXII that might be produced by contact activation of FXII in neat-buffer solution (such as FXIIf, see further ref. [17] and citations therein). Possible presence of activated forms other than FXIIa did not alter the basic conclusions of this work.
2.4 Contact Activation of Blood Plasma
The catalytic potential of an activator to induce plasma coagulation was assayed using the surface-area titration method reported previously [15, 20, 29-34]. Briefly, 0.5 mL of thawed plasma equilibrated with ambient temperature was transferred into 12 × 75 mm polystyrene tubes (VWR International, West Chester, PA) containing incrementally-increasing amounts of activator particles sufficient to cover the 0–25 cm2/mL surface-to-volume ratio when mixed with a total 1 mL test fluid obtained by adding 0.4mL of PBS and 0.1mL of 0.1M CaCl2 to the plasma. Tubes were immediately capped with parafilm and mounted on the table of a slowly-turning hematology mixer (approximately 4.5 revolutions/min, Roto-shake Genie, Scientific Industries, Inc.). Coagulation time after recalcification was noted by a distinct change in fluid-like rheology to gel formation, allowing determination of the end point of the coagulation process to within 10 s or so [15]. These simple, yet highly sensitive, recalcification-time assays eliminated extraneous contributions to coagulation associated with many modern instrumented tests (e.g. activating surfaces of stirrers and tubing) and yielded smooth dose-response curves (Fig. 1) that correlated with activator properties (surface chemistry and energy, see refs. [15, 20, 31, 32, 34]).
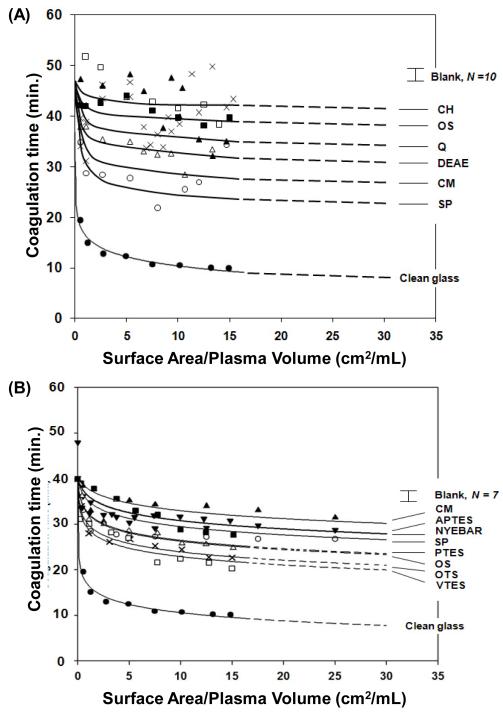
Figure 1.
Surface-area titration of blood plasma coagulation by (A) sepharose-based ion-exchange resin particles compared to clean-glass particles and (B) selected ion-exchange resin particles compared to silanized glass particles. Activator identity is given in Table 1. Ion-exchange resins are modest activators of plasma coagulation compared to clean glass, falling in a band that overlaps with activation properties of silanized glass particles incrementally sampling the observable surface-energy (water-wetting) range. The error bar labeled “Blank” represents the mean and standard deviation of N measurements of plasma coagulation in an empty tube. Smooth lines through the data represent best fit of a mathematical model of coagulation to the data.
Surface-area-titration data was fit to a theory described in ref. [15] designed to extract a parameter Kact that measured activator catalytic potential relative to an internal standard ( Kact has units of mL/m2 [31], not m−2 as reported in [15]). The standard of reference used herein was clean glass particles. A rate constant required by theory (designated as either kp in ref. [31] or k2 in ref. [15]) that measured the rate of fibrin polymerization (for a particular lot of plasma) was determined by a two-parameter fit (kp and Kact ) to the glass standard as described in refs. [15, 31], yielding kp =0.37± 0.4 min−1 (best fit value ± standard error of the fit, R2 = 99.7% ; compare to 0.54 ± 0.10 min−1 for human plasma from ref. [31] and 0.70 ± 0.09 min−1 for porcine plasma from ref. [15]). Thus, Kact measured activation potential relative to the glass standard and should not to be interpreted as a quantitative characteristic of procoagulants studied herein. Likewise kp should not be regarded as representative of human PPP in general (see ref. [15] for more discussion).
2.5 Blood Factor XII Activation in Neat Buffer Solution
All FXII activation-test solutions were prepared by mixing 20 μL of activator-particle suspension (~ 5 cm2 surface area; Section 2.1) with 200 μL of 31.8 μg/mL FXII in PBS so that the final FXII concentration was 30 μg/mL (nominal physiologic concentration). Timed activations within the time interval 0 < t < 30 min in 5 min intervals were performed at 37 °C in a water bath in 0.5 mL centrifuge microtubes (Eppendorf). Apparent FXIIa was measured at each time interval according to Section 2.3 using 100 μL test solution.
2.6 Lysozyme Displacement of FXIIa Putatively Bound to CM and DEAE Activator Particles
Two arbitrarily-designed protocols were used in an attempt to displace FXIIa putatively bound to CM and DEAE activator particles using lysozyme as a polar protein (pI = 11) with high adsorption affinity for ion-exchange adsorbents [1] (see further Sections 3.4 and 4.3). Protocol I FXII activation/displacement experiments were conducted as described in Section 2.5 for 10 min followed by addition of 10 μL of a 33.3 mg/mL hen egg-white lysozyme (HEWL Calibiochem, Gibbstown, NJ) concentrate to obtain a final concentration of 1.5 mg/mL HEWL mixed with FXII solution. Protocol II FXII activation experiments used 200 μL of 3.1 mg/mL HEWL displacement fluid to obtain a final concentration of 1.5 mg/mL HEWL mixed with FXII solution.
3.0 Results
3.1 Contact Activation of Plasma Coagulation
Fig. 1A,B collects ion-exchange resin surface-area titrations (Section 2.4) comparing reduction in plasma coagulation time (CT) due to contact with increasing surface area of the different activators listed in Table 1. Fig. 1A (plasma lot # 1) shows that all ion-exchange resins were inefficient activators relative to clean glass following the general trend Clean Glass>>SP>CM>DEAE>Q<OS~CH, based on the lowest asymptotic CT obtained. Fig. 1B (plasma lot #2) compares the most activating ion-exchange resins CM and SP to silane-treated glass-particle activators listed in Table 1.
Activation efficiency of ion-exchange particles was quantified by fitting data of Fig. 1 to a theory that allowed extraction of the Kact parameter that measured activator catalytic potential to induce plasma coagulation (see Section 2.5 and refs. [15, 31, 35]). Lines through the data of Fig. 1 represent best fit of theory to data. Column 6 of Table 1 under “Sepharose Based Resins” collects Kact obtained using separate trials in plasma lots I and II. Results were compared to Kact of silanized glass particles with different surface energy under “Treated Glass Particles” in Table 1 tested with plasma lot I. In certain circumstances, activation was so inefficient that only order-of-magnitude Kact estimates could be made from the data (listed in Table 1 with no R2 goodness-of-fit parameter). Activation of FXII by glass-particle activators was reported in ref. [17] and protein-adsorption properties compared in ref. [3]. Blood protein adsorption to OS and ion-exchange resins was reported in ref. [1].
3.2 Calcium Titration of Contact Activation
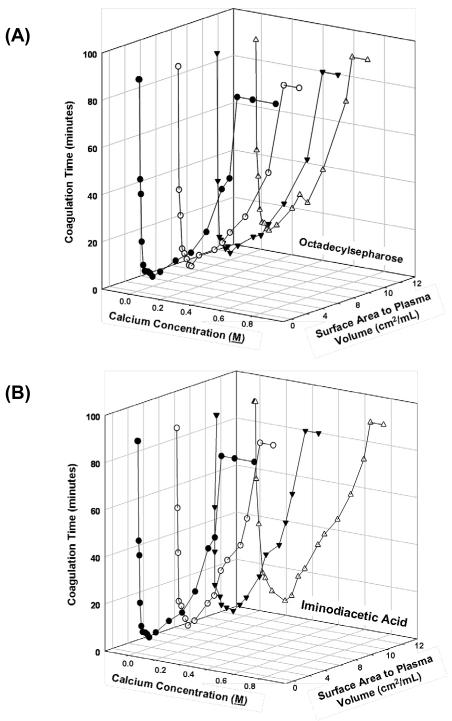
Figs. 2A,B compare surface area titrations performed at varying Ca+2 concentrations for octyl sepharose and iminodiacetic acid sepharose-based activators (Table 1). Generally speaking there was no substantial difference between these two activators. Nearly identical curves were obtained using CM (negative) or DEAE (positive) ion-exchange resins (not shown). In all cases, including glass-particle activators listed in Table 1, minimum coagulation time for all surface areas tested when citrated plasma was recalcified with 0.1 M CaCl2.
Figure 2.
Three-dimensional plots relating calcium concentration used to recalcify citrated plasma and surface area of sepharose-based activators for (A) hydrophobic octyl sepharose and (B) imidodiacetic acid. Similar results were obtained with other sepharose-based activators listed in Table 1 (not shown). Presence of ion-exchange resins did not measurably affect recalcification.
3.3 FXII Activation in Neat-Buffer Solution
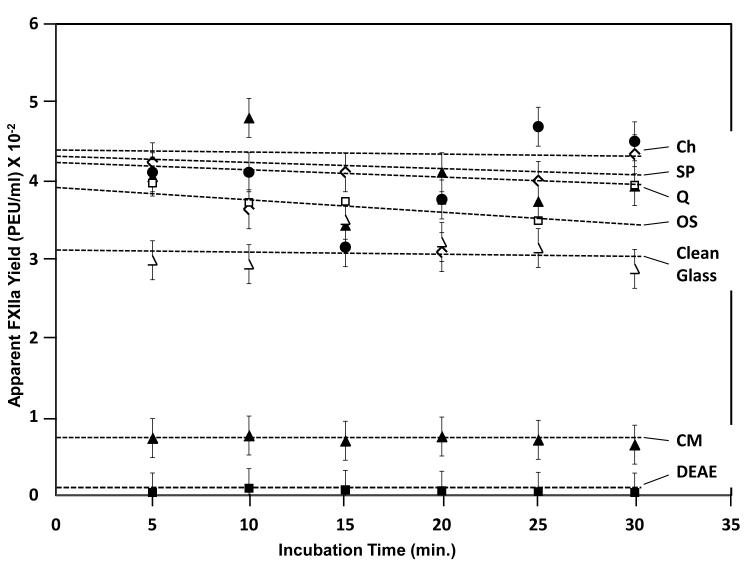
Data of Fig. 3 compares autoactivation of FXII by ion-exchange resins and clean glass activators from which it was evident that the order of autoactivation efficiency followed the apparent order CH~SP~Q>OS>Clean Glass>>CM>DEAE. This ordering was consistent with previous observations that hydrophobic activators (OS) were slightly more efficient than hydrophilic clean glass [17, 28]. Kinetic trends were statistically flat, consistent with our previous studies [16].
Figure 3.
Autoactivation of FXII in neat-buffer solution by sepharose-based ion-exchange resin particles listed in Table 1. Ion-exchange resins are more efficient activators than clean glass. CM and DEAE appear to be inefficient activators because FXIIa binds to the surface and is not detected by the FXIIa assay (see Fig. 4). Dashed lines through the data are guides to the eye.
3.4 Lysozyme Displacement of FXIIa Putatively Bound to CM and DEAE
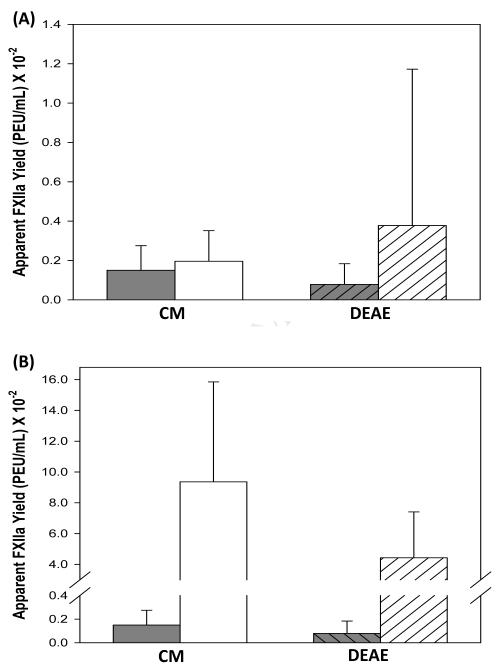
Figs. 4A,B compare yield of FXIIa obtained by activation with CM and DEAE in the presence of high concentration of HEWLS according to Protocol I and Protocol II of Section 2.6, respectively. The intention of these experiments was to displace FXIIa that might have become bound (strongly adsorbed) to these activator particles, causing CM and DEAE to appear to have lower autoactivation efficiency than other sepharose-based activators (see Fig. 3). Protocol I did not displace putatively-bound FXIIa whereas statistically significant amount of FXIIa was displaced from CM and DEAE by Protocol II.
Figure 4.
Desorption of FXIIa bound to CM and DEAE activators by (A) Protocol I (10 μL of 1.5 mg/mL lysozyme solution ) and (B) Protocol II (200 μL of 1.5 mg/mL lysozyme solution). Protocol I did not displace statistically-significant quantities of adsorbed FXIIa but a large and statistically-significant amount of FXIIa was displaced using a Protocol II.
4.0 Discussion
The significant finding of this work is that ion-exchange resins bearing strong Lewis acid/base functional groups efficiently autoactivate FXII in neat-buffer solution (Fig. 3) but do not efficiently contact activate blood plasma coagulation (Fig. 1); where activation efficiency is qualitatively compared to hydrophilic clean-glass particles, hydrophobic octyl sepharose, and a variety of silanized-glass-particle activators. These comparison materials have been extensively characterized in terms of FXII autoactivation, activation of plasma coagulation, and protein adsorption properties [2, 3, 11, 13, 17]. We interpret inefficient activation of plasma coagulation by ion-exchange particles in terms of the “adsorption dilution” phenomenon mentioned in Section 1 whereby adsorption of proteins unrelated to the plasma coagulation cascade effectively impedes protein-adsorbent surfaces from contact with FXII, causing low autoactivation yield, and consequently sluggish plasma coagulation in contact with protein-adsorbent surfaces. The following subsections discuss experimental findings supporting this interpretation in order of presentation in Section 3. We end by putting conclusions into the overall context of blood plasma coagulation relevant to development of cardiovascular biomaterials.
4.1 Contact Activation of Plasma Coagulation
Surface-area titration curves like those shown in Fig. 1 measure the potential of a material surface to contact activate blood-plasma coagulation. These simple functional assays exhibit a sharp surface-area dependence that exponentially dampens to a lower-CT asymptote that depends on both activator surface chemistry, energy, and nanoscopic distribution of chemistry on the activator surface [15, 20, 29, 31]. Clean glass is among the class of “anionic hydrophilic” activators that is well known to strongly potentiate plasma coagulation [16].
The rate-of-change in CT with surface area is a measure of the activation efficiency of test materials. It is somewhat surprising to observe in this regard that both acid and base ion-exchange resins are relatively inefficient activators of plasma coagulation compared to clean glass (Fig. 1A), falling in a general band of activator properties with silanized glass particles bearing surface-functional groups that do not exhibit ion-exchange type properties (Fig. 1B). Given the Lewis acid/base strength and hydrophilicity of ion-exchange functionalities and the strong electric field surrounding these moieties [19], it might otherwise have been anticipated that ion-exchange surfaces would be among the most efficient, not inefficient, of activating materials. Ratnoff et al. noted in 1961 that carboxymethyl cellulose (CMC) activated plasma coagulation in a manner similar to glass [36], which over states CM activation compared to conclusions drawn from Fig. 1. But CMC is not chemically identical to the CM sepharose-based activators used herein and Ratnoff’s procedures were not the same as the surface-area titration of Section 2.4 employed herein. All issues taken into account leads us to conclude that our results are at least consistent with that of Ratnoff in that CM activates plasma coagulation, with the important caveat that CM is not as efficient in activating plasma coagulation as clean glass.
Plasma coagulation activation by sepharose-based activators and silanized-glass activators of Fig. 1 cannot be quantitatively compared due to uncertainty in relative dispensed surface area mentioned in Section 2.1. However, trends among these two groups of activators can be quantified by fitting data to a mathematical model that allows extraction of an adjustable parameter Kact that measures catalytic potential to activate plasma coagulation [15, 20, 31, 34]. Column 6 of Table 1 collects Kact for the activators compared in Fig. 1. Inspection of this data shows that catalytic potential of ion-exchange resins are no more than an order-of-magnitude more activating than nearly inert hydrophobic OS. Treated-glass particles exhibit a broad range Kact values that depend on activator surface energy [15, 17, 31, 34]. Again bearing in mind that direct comparison of Kact between these two groups of activators is problematic, face-value interpretation shows that sepharose-based activators are between two-or-three decades less activating than clean glass and a decade less activating than hydrophobic silanized glass (OTS). From this perspective, low catalytic potential of ion-exchange resins is perhaps even more surprising.
4.2 Calcium Titration of Contact Activation
Recalcification of citrated plasma is a traditional tool used in coagulopathy testing and hematological research [26]. It is well known that CT of recalcified plasma is very sensitive to calcium-ion concentration and it is therefore reasonable to suggest that presence of ion-exchange resins in recalcified plasma might significantly affect calcium concentration and observed CT. Fig. 2A,B shows that CT is indeed sensitive to calcium concentration, falling sharply through a minimum obtained at 0.1 M CaCl2 and rising through 1.0 M CaCl2. Fig. 2A compares the affect of added surface area of neutral OS (no ion-exchange capacity) on the calcium titration curve to that of CH “chelating” resin in Fig. 2B (see Table 1). Similar curves were obtained for other ion-exchange resins listed in Table 1 (not shown). There was no measurable effect of activator particle surface area over the range explored in Fig. 1, showing that modest plasma activation by ion-exchange resins cannot be attributed to calcium concentration in the recalcification CT assay.
4.3 FXII Activation in Neat-Buffer Solution
Fig. 3 compares autoactivation of FXII in neat-buffer solution by sepharose-based activators to clean glass. The apparent yield following CH~SP~Q>OS>glass>>CM>DEAE must be interpreted in light of uncertainty in dispensed surface area mentioned in Section 2.1 and protein-adsorption properties of sepharose-based activators compared to clean glass. It is possible that if dispensed surface area of glass was significantly lower than sepharose-based activators, then real autoactivation of glass might appear higher in the apparent order when corrected for surface area, which is otherwise assumed to be the same between activator types. But there can be little doubt that the order for sepharose-based activators would rise relative to glass when the fact that an unknown amount of both FXII and FXIIa is undoubtedly adsorbed to sepharose-based activator. We have shown that ion-exchange surfaces have a high protein capacity compared to hydrophobic OS [1] and that protein does not adsorb to hydrophilic glass [2-5]. We conclude, therefore, that sepharose-based activators are at least as efficient in activating FXII in neat-buffer solution as clean glass and most likely much more efficient when protein adsorption is taken into account.
The comparison of autoactivation efficiency within sepharose-based activators CH~SP~Q>OS>>CM>DEAE is not affected by surface area consideration in so far as these particles have similar specific surface area. It is surprising in this regard that CM and DEAE are so much less efficient in activation of FXII. This turns out to be due to the fact that FXIIa is very strongly bound to these activators and is unavailable in solution to be detected by the detected by the FXIIa assay of Section 2.3, as discussed in the following subsection.
4.4 Lysozyme Displacement of Adsorbed FXIIa
Lysozyme is a small (MW = 10 kDa) polar protein (pI = 11) with high adsorption affinity for ion-exchange adsorbents that has been used to displace higher-MW proteins from ion-exchange surfaces [1]. We used lysozyme displacement as a probe for FXIIa strongly adsorbed to CM and DEAE activators. As summarized in Fig. 4A, Protocol I (Section 2.6) was insufficient to displace statistically-significant quantities of FXIIa putatively adsorbed to either CM or DEAE. However, a large and statistically-significant amount of FXIIa was displaced using Protocol II using the same lysozyme concentration at high volume. We interpret these findings to mean that high relative concentration of lysozyme alone was insufficient to displace bound FXIIa (Protocol I) but that both high relative lysozyme concentration and high dilution (Protocol II) was required to displace surface-bound FXIIa. High dilution creates a significant concentration gradient between the surface region (a.k.a interphase) and bulk solution causing FXIIa to partition out of the interphase into solution (see refs. [11, 18] for a discussion of interphase and protein partitioning between bulk solution and interphase). These experimental results are consistent with the early work of Ratnoff and Davie who used strong FXIIa binding to carboxymethyl (CM) cellulose and DEAE-cellulose as the basis of chromatographic purification of active FXIIa [37].
4.5 Collective Interpretation
Two striking observations come forward from a comparison of Fig. 3 and Fig. 1A and in consideration of the discussion in the preceding two sections. First, it is evident that although CH, SP, Q, and OS more efficiently activate FXII in neat-buffer solution than clean glass (Fig. 3), these sepharose-based activators are not as efficient in activating plasma coagulation as clean glass (Fig. 1A). This is unusual because efficient autoactivation turns out not to be directly linked to efficient activation of plasma coagulation, which otherwise is the expectation based on the known biochemistry of the coagulation cascade [16]. The second observation is a reverse of the first; although CM and DEAE were among the most potent sepharose-based activators of plasma coagulation (Fig. 1A), these particular activators were the least efficient activators of FXII in neat-buffer solution (Fig. 3).
The first observation can be rationalized by invoking the “adsorption-dilution effect” mentioned in Section 1 and discussed at length in refs. [16-18]. CH, SP, Q, and OS are known to efficiently adsorb blood proteins [1, 2, 11, 13]. In fact, the protein-adsorption capacity of CH, SP, and Q is much higher than that of hydrophobic OS [1]. This extraordinary adsorbent capacity is related to strong protein/surface interactions mediated by the electric field surrounding ion-exchange resins [1, 19]. Thus, even though CH, SP, Q, and OS efficiently autoactivate FXII in neat-buffer solution, activation of plasma coagulation is muted by wholesale adsorption of plasma proteins that compete with FXII/surface contacts. Adsorption competition impedes surface-FXII interactions, causing low autoactivation yield and consequently sluggish coagulation.
The second observation can be explained by strong binding of FXIIa to CM and DEAE surfaces that prevents release into solution where it can be detected by the FXIIa assay of Section 2.3. Hence CM and DEAE only appear to inefficiently autoactivate FXII in neat-buffer solution (Fig. 3) when, in fact, CM and DEAE are efficient contact activators of FXII. Lysozyme displacement discussed in Section 4.4 proves that FXIIa can be strongly bound to ion-exchange surfaces. A similar FXIIa-displacement effect can be anticipated to occur in the plasma-coagulation assay used in collection of data of Fig. 1 due to the high concentration of plasma proteins (50% plasma is approximately 25 mg/mL total protein). Hence, even though CM and DEAE strongly bind FXIIa, these particles activate plasma coagulation because some-or-all of the FXIIa produced by autoactivation is displaced by adsorption competition with plasma proteins. Displaced FXIIa becomes available in solution and potentiates the plasma-coagulation cascade. It is also possible that bound FXIIa activates plasma coagulation, although we were unable to demonstrate this (not shown) using methods described by Zhuo et al. who measured FXIIa activity bound to hydrophobic activators [28].
All evidence taken together, we conclude that sepharose-based activators listed in Table 1 efficiently autoactivate FXII. Ion-exchange activation of plasma coagulation is lower than glass because of the adsorption-dilution effect that impedes FXII/surface interactions with ion-exchange surfaces. Although this rationalization of experimental results explains the qualitative relationships observed between autoactivation and plasma coagulation activation, it does not immediately explain the ordering of plasma activation in Fig. 1A (SP>CM>DEAE>Q<OS~CH) in relation to the ordering in autoactivation efficiency from Fig. 3 (CH~SP~Q>OS >>CM>DEAE). Results of Fig. 4 suggest CM and DEAE are actually very efficient activators of FXII in plasma and that the autoactivation order is possibly more like CM>DEAE>CH~SP~Q>OS when corrected for FXIIa binding to CM and DEAE surfaces. This sorting more closely emulates the plasma coagulation order but is not a match. A finer re-sorting would require a means of assessing autoactivation efficiency independent of FXIIa binding and a measure of FXIIa binding energies to the different ion-exchange particles.
4.6 Contact Inhibition of Coagulation Factors
Surface functionalization with strong anionic and cationic chemistries has been used for years in the preparation of heparized materials for blood-contact applications (refs. [38-40] are early citations from which a vast literature has arisen). In studies of various chemistries available for heparin immobilization, it was discovered that certain blood coagulation factors (such as FXII) became bound to treated surfaces [38] and that activity of other factors such as IX, X, and II was reduced, especially by contact with cationic surfaces [39] (where factor activity was typically measured by conventional thrombin time, prothrombin time, and partial thromboplastin time assays). A recently issued patent [41] claims that factors V and VIII are inactivated by contact with surfaces bearing an iminodiacetic acid group (designated CH herein). Thus, a possible explanation for the modest plasma-coagulation-activation efficiency by sepharose-based activators shown in Fig. 1A is that coagulation factors are inactivated or inhibited by contact with these surfaces. Accordingly, efficient FXII autoactivation by sepharose-based activators does not lead to rapid plasma coagulation because procoagulant stimulus is not efficiently propagated down the coagulation cascade. This explanation presents itself as an alternative to the adsorption-dilution effect proposed in the preceding section.
This work did not measure activity of individual coagulation factors other than FXIIa and consequently cannot directly corroborate or dismiss the factor inactivation/inhibition proposal. However, there are at least five reasons that suggest coagulation factor inactivation/inhibition alone is insufficient to explain inefficient plasma activation by sepharose-based activators. First, it is evident from Fig. 1A that none of the coagulation factors were inactivated by contact with sepharose-based activators, each being critical to propagation of contact activation stimulus through the intrinsic pathway of plasma coagulation [16]. In particular, there are no known pathways circumventing the tenase complex or production of thrombin (FIIa). Observation of coagulation thus insures at least partial activity of each factor, especially that of FII, IX, or FX. Second, the factor inhibition explanation requires an unlikely proportional increase in inhibition with increasing autoactivation. That is, in order to explain trends in the data of Fig. 1A, inhibition must increase with increasing surface area of sepharose-based activators, counterbalancing increasing production of FXIIa by autoactivation (autoactivation is surface-area dependent [18, 28, 31]). In fact, this balance between autoactivation and factor inhibition must just happen to occur in such a way that surface-area titration of sepharose-based activators produces nested curves like that obtained for silanized glass (Fig. 1B), self-assembled monolayer, and oxidized-polymer activators (see ref. [15]). In the case of these latter particulate activators, factor inhibition is neither anticipated nor necessary to explain the dampening-exponential shape of surface-titration curves (see refs. [15, 31, 34] for mathematical models of the contact activation of plasma coagulation). Third, factor inhibition alone seems unlikely to significantly modulate coagulation clot time in a surface-area titration such as shown in Fig. 1 because only a small portion of available zymogens is actually activated by contact activation [34]. For examples, we find that only about 20% of FI (fibrinogen) is converted to fibrin at coagulation time in surface-titrations like that of Fig. 1 [29], strong autoactivation converts less than about 3% of available FXII to FXIIa [17] which is amplified by no more than 4x through the kallikrein-mediated reciprocal loop [30], and that a small portion of FII is converted to FIIa [16] in bolus proportion to the intensity of contact activation [31]. So, it seems that there is much more zymogen in plasma than necessary for a fully-functional cascade and that inhibition of one or more of the factors would not significantly delay coagulation. Fourth, as for factors FV and VIII, neither are principle zymogens of the plasma coagulation cascade; FV is a co-factor that binds to activated platelets and FVIII is a co-factor involved in the tenase complex that is itself activated by FIIa. Thus it would appear that inhibition of these factors is not important in surface-contact activation of platelet-poor plasma coagulation through the intrinsic pathway. Fifth and finally, the adsorption-dilution phenomena itself argues that the contact frequency of coagulation factors at less than μg/mL solution concentration with ion-exchange surfaces in the presence of 25 mg/mL plasma proteins must be quite low. We observe, for example, that efficient FXII activation in neat-buffer by hydrophobic particles [28] is nearly eliminated in the presence of protein cocktails [18] or plasma proteins [17]. Thus, contact of coagulation factors by ion-exchange surfaces with even higher protein-adsorbent capacity [1] must be correspondingly low. Based on these reasons we conclude that plasma proteins play the principle role in moderating activation of plasma coagulation by sepharose-based activators through the adsorption-dilution effect and that factor inhibition plays a secondary role, if it occurs at all for the materials studied herein.
4.7 Implications for Cardiovascular Biomaterials and Understanding Hemocompatibility
Ion-exchange resins bearing strong Lewis acid/base functional groups efficiently activate FXII in comparison to clean glass which is well known to efficiently activate plasma coagulation [16]. Thus, it seems unlikely that these materials will be useful in the development of blood-contacting cardiovascular biomaterials but ion-exchange chemistries will continue to have utility in the separation and purification of blood factors (see, for example, refs. [37, 42, 43]).
Comparison of autoactivation of FXII in neat-buffer solution to activation of plasma coagulation by ion-exchange resin particles reveals subtle details of the role of plasma proteins in moderating the plasma coagulation cascade [16, 17]. Proteins unrelated to the plasma coagulation cascade at high plasma concentrations such as serum albumin (66.3 kDa, ~45 mg/mL), immunoglobulin G (160 kDa, ~10 mg/mL), and transferrin (77 kDa, ~3 mg/mL) moderate plasma coagulation not only by impeding blood zymogen/surface interactions (the adsorption-dilution effect [16-18]) but also by competitively desorbing activated factors from activator surfaces. This dual role of plasma proteins becomes evident in the case of ion-exchange activators because both autoactivation efficiency and protein-adsorption capacity is higher than for hydrophobic activators such as OS or silanized glass. Increased FXII adsorption by ion-exchange surfaces, even in competition with a plethora of plasma proteins, combined with high autoactivation efficiency produces significant FXIIa. As-produced FXIIa is then desorbed from protein-adsorbent ion-exchange surfaces by adsorption competition with plasma proteins. Thus it happens that materials that are both efficient activators of FXII and protein adsorbents exhibit intermediate catalytic potential to activate plasma coagulation resembling that of ordinary materials (such as silanized glass activators). By contrast, plasma proteins do not moderate activation of plasma coagulation by hydrophilic activators such as clean glass because these activators do not adsorb plasma proteins (see, for examples, refs. [2, 3, 5, 20, 21] and citations therein). Hence clean-glass activator particles are more efficient activators of plasma coagulation even though autoactivation of FXII by glass particles is less efficient than either hydrophobic or sepharose-based ion-exchange activators. This proposed mechanism is consistent with the mathematical model of autoactivation proposed Zhuo et al. that successfully accounted for autoactivation kinetics and steady-state yield of FXIIa [18].
5.0 Conclusions
Surfaces bearing strong Lewis acid/base functional groups represented by anion and cation ion-exchange particles are efficient contact activators of blood factor XII (FXII, Hageman factor), the first step of the intrinsic pathway of blood plasma coagulation [16]. These resin particles are also very efficient protein adsorbents [1]. As a consequence of these two special properties, contact of ion-exchange particles with FXII in plasma is substantially impeded by adsorption of a plethora plasma proteins through a mechanism that has been termed an “adsorption-dilution effect” [16] operating in a manner paralleling that observed for hydrophobic activators bearing no Lewis acid/base functional groups. Activation of plasma coagulation by ion-exchange resins is thus observed to be much less efficient than hydrophilic glass that does not adsorb proteins from plasma. The graded plasma-activation properties observed among different ion-exchange chemistries can be qualitatively understood on the basis of FXII activation potential and desorption of FXIIa from the ion-exchange surfaces by competitive adsorption of plasma proteins that displace surface-bound FXIIa. An adequate description of the mechanism of contact activation of blood plasma coagulation must include the participation of proteins unrelated to coagulation through competitive adsorption to activator surfaces. These results require a reassessment of how the intrinsic pathway of blood-plasma processes activator stimulus and provokes a revision of our traditional understanding of hemocompatibility [18, 31].
Impact Statement.
This work strongly suggests that the traditional understanding of hemocompatibility must be revised to accommodate an important role of plasma proteins in moderating contact activation of blood plasma coagulation.
Acknowledgments
This work was supported the National Institute of Health grant PHS 2R01HL069965. The authors are grateful for support from the Department of Materials Science and Engineering and Bioengineering of The Pennsylvania State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- [1].Noh H, Vogler EA. Volumetric interpretation of protein adsorption: ion-exchange adsorbent capacity, protein pI, and interaction energetics. Biomaterials. 2008;29:2033–48. doi: 10.1016/j.biomaterials.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Noh H, Vogler EA. Volumetric interpretation of protein adsorption: mass and energy balance for albumin adsorption to particulate adsorbents with incrementally-increasing hydrophilicity. Biomaterials. 2006;27:5801–12. doi: 10.1016/j.biomaterials.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [3].Parhi P, Golas A, Barnthip N, Vogler EA. Volumetric interpretation of protein adsorption: capacity scaling with adsorbate molecular weight and adsorbent surface energy. Biomaterials. 2009;30:6814–24. doi: 10.1016/j.biomaterials.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cha P, Krishnan A, Fiore VF, Vogler EA. Interfacial energetics of protein adsorption from aqueous buffer to surfaces with varying hydrophilicity. Langmuir. 2008;24:2553–63. doi: 10.1021/la703310k. [DOI] [PubMed] [Google Scholar]
- [5].Vogler EA, Martin DA, Montgomery DB, Graper JC, Sugg HW. A graphical method for predicting protein and surfactant adsorption properties. Langmuir. 1993;9:497–507. [Google Scholar]
- [6].Krishnan A, Sturgeon J, Siedlecki CA, Vogler EA. Scaled interfacial activity of proteins at the liquid-vapor interface. J Biomed Mat Res. 2004;68A:544–57. doi: 10.1002/jbm.a.20104. [DOI] [PubMed] [Google Scholar]
- [7].Krishnan A, Siedlecki C, Vogler EA. Traube-rule interpretation of protein adsorption to the liquid-vapor interface. Langmuir. 2003;19:10342–52. [Google Scholar]
- [8].Krishnan A, Wilson A, Sturgeon J, Siedlecki CA, Vogler EA. Liquid-vapor interfacial tension of blood plasma, serum and purified protein constituents thereof. Biomaterials. 2005;26:3445–53. doi: 10.1016/j.biomaterials.2004.09.016. [DOI] [PubMed] [Google Scholar]
- [9].Krishnan A, Liu Y-H, Cha P, Allara DL, Vogler EA. Interfacial energetics of globular-blood protein adsorption to a hydrophobic surface from aqueous-buffer solution. J Roy Soc Interface. 2006;3:283–301. doi: 10.1098/rsif.2005.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Krishnan A, Liu Y-H, Cha P, Allara DL, Vogler EA. Scaled Interfacial activity of proteins at a hydrophobic solid/aqueous-buffer interface. J Biomed Mater Res. 2005;75A:445–57. doi: 10.1002/jbm.a.30444. [DOI] [PubMed] [Google Scholar]
- [11].Noh H, Vogler EA. Volumetric interpretation of protein adsorption: partition coefficients, interphase volumes, and free energies of adsorption to hydrophobic surfaces. Biomaterials. 2006;27:5780–93. doi: 10.1016/j.biomaterials.2006.07.038. [DOI] [PubMed] [Google Scholar]
- [12].Krishnan A, Liu Y-H, Cha P, Allara DL, Vogler EA. Interfacial energetics of blood plasma and serum adsorption to a hydrophobic self-assembled monolayer surface. Biomaterials. 2006;27:3187–94. doi: 10.1016/j.biomaterials.2005.12.032. [DOI] [PubMed] [Google Scholar]
- [13].Noh H, Vogler EA. Volumetric interpretation of protein adsorption: competition from mixtures and the Vroman effect. Biomaterials. 2007;28:405–22. doi: 10.1016/j.biomaterials.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vroman L. What factors determine thrombogenicity? Bull N Y Acad Med. 1972;48:302–10. [PMC free article] [PubMed] [Google Scholar]
- [15].Vogler EA, Graper JC, Harper GR, Lander LM, Brittain WJ. Contact activation of the plasma coagulation cascade.1. procoagulant surface energy and chemistry. J Biomed Mat Res. 1995;29:1005–16. doi: 10.1002/jbm.820290813. [DOI] [PubMed] [Google Scholar]
- [16].Vogler EA, Siedlecki CA. Contact activation of blood plasma coagulation. Biomaterials. 2009;30:1857–69. doi: 10.1016/j.biomaterials.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Golas A, Parhi P, Dimachkie ZO, Siedlecki CA, Vogler EA. Surface-energy dependent contact activation of blood factor XII. Biomaterials. 2010;31:1068–79. doi: 10.1016/j.biomaterials.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhuo R, Siedlecki CA, Vogler EA. Competitive-protein adsorption in contact activation of blood factor XII. Biomaterials. 2007;28:4355–69. doi: 10.1016/j.biomaterials.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xu X, Lenhoff AM. A predictive approach to correlating protein adsorption isotherms on ion-exchange media. J Phys Chem B. 2008 doi: 10.1021/jp0754233. [DOI] [PubMed] [Google Scholar]
- [20].Vogler EA, Graper JC, Sugg HW, Lander LM, Brittain WJ. Contact activation of the plasma coagulation cascade.2. protein adsorption on procoagulant surfaces. J Biomed Mat Res. 1995;29:1017–28. doi: 10.1002/jbm.820290814. [DOI] [PubMed] [Google Scholar]
- [21].Vogler EA. Practical use of concentration-dependent contact angles as a measure of solid-liquid adsorption II: Experimental Aspects. Langmuir. 1992;8:2013–20. [Google Scholar]
- [22].Friberger P. Synthetic peptide substrate assays and fibrinolysis and their application on automates. seminars in thrombosis and haemostasis. 1983;9:281–300. [PubMed] [Google Scholar]
- [23].Woodhams B, Girardot O, Blanco MJ, Gourmelin Y. Stability of coagulation proteins in frozen plasma. Blood Coagul Fibrin. 2001;12:229–36. doi: 10.1097/00001721-200106000-00002. [DOI] [PubMed] [Google Scholar]
- [24].Alesci S, Borggrefe M, Dempfle CE. Effect of freezing method and storage at-20 degrees C and-70 degrees C on prothrombin time, aPTT and plasma fibrinogen levels. Thromb Res. 2009;124:121–6. doi: 10.1016/j.thromres.2008.11.010. [DOI] [PubMed] [Google Scholar]
- [25].Lamboo M, Poland DCW, Eikenboom JCJ, Harvey MS, Groot E, Brand A, et al. Coagulation parameters of thawed fresh-frozen plasma during storage at different temperatures. Transfusion Med. 2007;17:182–6. doi: 10.1111/j.1365-3148.2007.00729.x. [DOI] [PubMed] [Google Scholar]
- [26].Brown B. Hematology: principles and procedures. 3 ed Lea and Febiger; Philadelphia: 1980. [Google Scholar]
- [27].Zhuo R, Vogler EA. Practical application of a chromogenic FXIIA assay. Biomaterials. 2006;27:4840–5. doi: 10.1016/j.biomaterials.2006.05.008. [DOI] [PubMed] [Google Scholar]
- [28].Zhuo R, Siedlecki CA, Vogler EA. Autoactivation of blood factor XII at hydrophilic and hydrophobic surfaces. Biomaterials. 2006;27:4325–32. doi: 10.1016/j.biomaterials.2006.04.001. [DOI] [PubMed] [Google Scholar]
- [29].Vogler EA, Nadeau JG, Graper JC. Contact activation of the plasma coagulation cascade. 3. biophysical aspects of thrombin binding anticoagulants. J Biomed Mat Res. 1997;40:92–103. doi: 10.1002/(sici)1097-4636(199804)40:1<92::aid-jbm11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [30].Chatterjee K, Guo Z, Vogler EA, Siedlecki CA. Contributions of contact activation pathways of coagulation factor XII in plasma. J Biomed Mat Res - A. 2009;90A:27–34. doi: 10.1002/jbm.a.32076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhuo R, Miller R, Bussard KM, Siedlecki CA, Vogler EA. Procoagulant stimulus processing by the intrinsic pathway of blood plasma coagulation. Biomaterials. 2005;26:2965–73. doi: 10.1016/j.biomaterials.2004.08.008. [DOI] [PubMed] [Google Scholar]
- [32].Miller R, Guo Z, Vogler EA, Siedlecki CA. Plasma coagulation response to surfaces with nanoscale heterogeneity. Biomaterials. 2006;27:208–15. doi: 10.1016/j.biomaterials.2005.05.087. [DOI] [PubMed] [Google Scholar]
- [33].Chatterjee K, Vogler EA, Siedlecki CA. Procoagulant activity of surface-immobilized hageman factor. Biomaterials. 2006;27:5643–50. doi: 10.1016/j.biomaterials.2006.07.025. [DOI] [PubMed] [Google Scholar]
- [34].Guo Z, Bussard K, Vogler EA, Siedlecki CA. Mathematical modeling of material-induced blood plasma coagulation. Biomaterials. 2006;27:796–806. doi: 10.1016/j.biomaterials.2005.06.021. [DOI] [PubMed] [Google Scholar]
- [35].Zhuo R, Colombo P, Pantano C, Vogler EA. Silicon oxycarbide glasses for blood-contact applications. acta Biomaterialia. 2005;1:583–9. doi: 10.1016/j.actbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- [36].Ratnoff OD, Davie EW, Mallett DL. Studies on the action of hageman factor: evidence that activated hageman factor in turn activates plasma thromboplastin antecedent. J Clin Invest. 1961;40:803–19. doi: 10.1172/JCI104314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ratnoff OS, Davie EW. The purification of activated hageman factor. Biochemistry. 1962;6:967–74. doi: 10.1021/bi00912a005. [DOI] [PubMed] [Google Scholar]
- [38].Brenner WI, Engelman RM, Williams CD, Boyd AD, Reed GE. Nonthrombogenic aortic and vena caval bypass using heparin-coated tubes. The Am J Surg. 1974;127:555–9. doi: 10.1016/0002-9610(74)90316-x. [DOI] [PubMed] [Google Scholar]
- [39].Oja PD, Holmes GW, Perkins HA, Love J. Specific coagulation factor adsorption as related to functional groups on surfaces. J Biomed Mat Res. 1969;3:165–74. doi: 10.1002/jbm.820030113. [DOI] [PubMed] [Google Scholar]
- [40].Gott VL, Whiffen JD, Valiathan SM. Graphite-benzalkonium-heparin coatings on plastics and metals. Ann N Y Acad of Sci. 1968;146:21–9. doi: 10.1111/j.1749-6632.1968.tb20268.x. [DOI] [PubMed] [Google Scholar]
- [41].Okuda M, Yamamoto Y, Yoshioka A, Shima M, Takeyama M. Method of inactivating blood coagulation factor and blood coagulation factor-inactivated sample. US7264936: Sysmex Corporation. 2007 [Google Scholar]
- [42].Bouma BN, Griffin JH. Human blood coagulation factor XI. purification, properties, and mechanism of activation by activated factor XII. J Biol Chem. 1977;252:6432–7. [PubMed] [Google Scholar]
- [43].Fischer B, Mitterer A, Dorner F. Purification of recombinant human coagulation factors II and IX and protein s expressed in recombinant vaccinia virus-infected vero cells. J Biotechnol. 1995;38:129–36. doi: 10.1016/0168-1656(94)00124-u. [DOI] [PubMed] [Google Scholar]