Figure 2. TGF-β-LTBP assembly, processing, and activation.
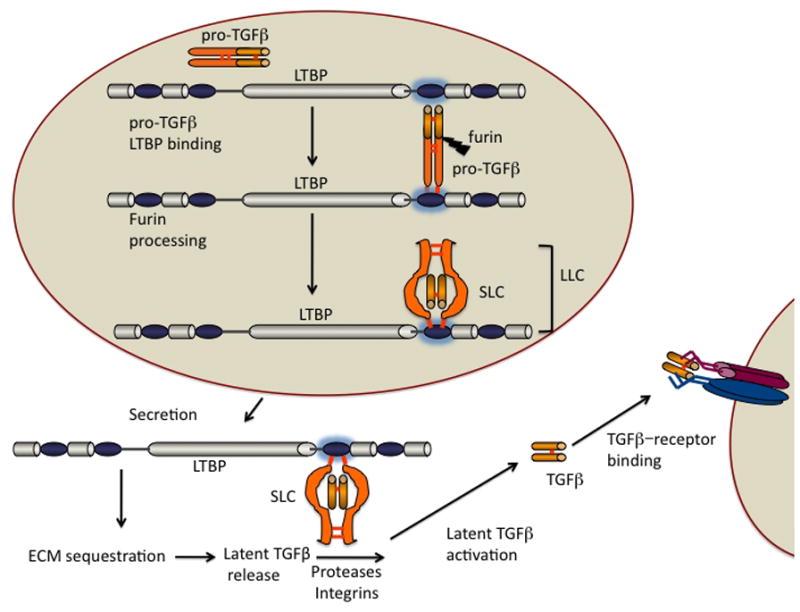
After dimerization in the endoplasmic reticulum, pro-TGFs molecules form disulfide bonds to LTBP molecules early in the secretory pathway. In the trans-Golgi, furin-like enzymes process the pro-TGF-β yielding mature TGF-β, the TGFβ propeptide (LAP), and an LTBP. This complex is called the large latent complex (LLC). When complexed with LAP, TGF-β cannot bind to its receptor. The LLC is secreted, binds to matrix components, and the latent TGF-β is activated by proteases/integrins that modify LAP or/and LTBP. The liberated TGF-β then binds to its receptor.
