Figure 6.
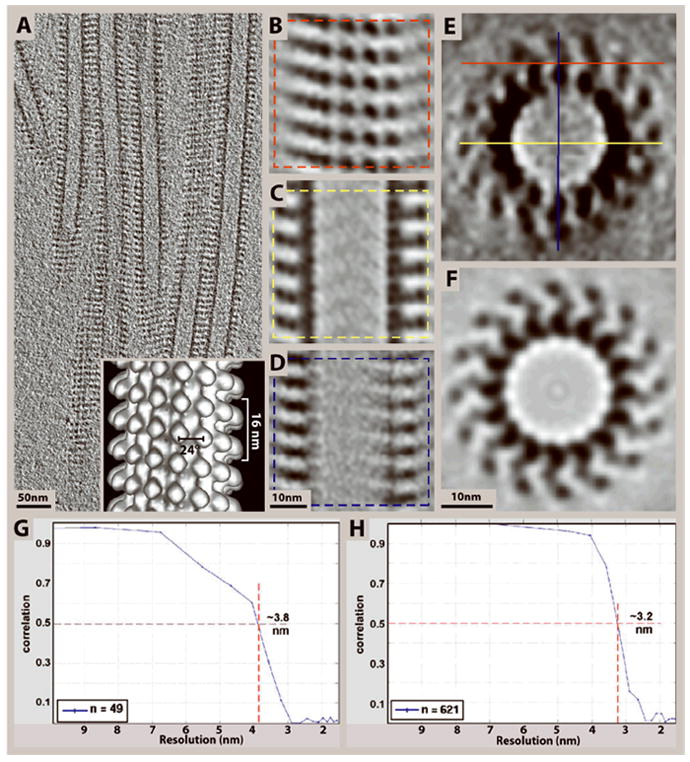
Incorporating symmetry with subtomogram averaging can effectively eliminate missing-wedge effects as demonstrated here on kinesin–microtubule complexes (Cope et al., 2010). (A) 10 nm slice through a cryo-tomogram of microtubules decorated with the heterodimeric kinesin Kar3Vik1. (B-D) 4 nm slices through the top and center of an average of 99 subvolumes selected from the tomogram in (A) according to the planes shown in (E). The central x-y slice in (C) is much less affected by the missing wedge, and shows well-defined densities corresponding to the α- and β- subunits of tubulin and the two globular domains of Kar3Vik1. (Only one of the two domains actually binds to the tubulin protofilaments). The central slice in (D) illustrates the loss of resolution along the Z-axis due to the missing wedge as compared to the x-y slice in (C). (E-F) A slice through the cross-section of the subvolume average before (E), and after rotational averaging (F), showing that rotational averaging can effectively eliminate missing-wedge effects. By averaging over all 16 protofilaments the asymmetric unit changes from axial slices along the microtubule to one αβ-tubulin–kinesin complex. Hence, the theoretical maximum number of asymmetric units included in the average is increased from 99 to 1584 (16 × 99). (Though our average is based on the 1242 particles with the best correlation to the reference because this subset gave the highest resolution). (G-H) For Fourier-shell correlation calculations, the datasets were split in half on a random basis and the two halves were correlated against each other. Fourier-shell correlation graphs obtained from the averages in E (G) and F (H) reveal resolution limits of 3.8 nm and 3.2 nm, respectively, based on the 50% correlation criterion. Inset in (A) shows a surface rendering of an average of a 15-protofilament microtubule after symmetrization as described in Supporting Protocol 1, step 3.
