Abstract
Background
Early disruption of the blood-brain barrier (BBB) due to severe ischemia can be detected by MRI T2* permeability imaging. In middle cerebral artery (MCA) infarction, pretreatment T2* permeability derangements have been found in 22% of patients and are powerful predictors of hemorrhagic transformation after revascularization therapy. The frequency, clinical correlates, and relation to hemorrhagic transformation of permeability derangements in posterior circulation have not been previously explored, and may differ as ischemia volume and collateral status are different between vertebrobasilar and MCA infarcts.
Methods
We analyzed clinical and pretreatment MRI data on consecutive patients undergoing recanalization therapy for acute vertebrobasilar ischemia at a medical center November 2001 through September 2009. Pretreatment MRI permeability images were derived from perfusion source imaging acquisitions. Permeability abnormality was detected as persisting increased signal intensity at later time points in perfusion MRI acquisition, indicating local accumulation of contrast caused by BBB leakage.
Results
Among the 14 patients meeting study entry criteria, mean age was 71.1 years and median pretreatment NIHSS was 20.5. Permeability imaging abnormality was present in 1 of the 14 patients (7%). Among 14 patients, post-treatment parenchymal hematoma occurred in one and more minor degrees of hemorrhagic transformation in four. The one patient with pretreatment permeability abnormality was the patient to develop post-treatment parenchymal hematoma (Fisher's exact test, P=0.07).
Conclusion
Pretreatment permeability abnormality, an indicator of BBB derangements, is an infrequent finding in acute posterior circulation ischemic stroke and may be associated with an increased risk of parenchymal hematoma development undergoing recanalization therapy.
Keywords: blood-brain barrier, basilar artery occlusion, posterior circulation ischemia, hemorrhagic transformation, recanalization
Hemorrhagic transformation is a feared complication of recanalization therapy for acute ischemic stroke. Established clinical and laboratory predictors of hemorrhagic transformation in patients with ischemic stroke include greater neurologic deficit severity, hyperglycemia, and later time to treatment. Some studies have already identified clinical and laboratory predictors of hemorrhagic transformation after recanalization therapy for stroke.1-5 Increasing use of multimodal magnetic resonance imaging (MRI) offers an additional source of data regarding tissue status and potential tendency for hemorrhagic transformation.6, 7 Several pretreatment MRI parameters have variably been reported in association with an increased risk of hemorrhagic transformation after recanalization therapy: leukoaraiosis,8 prior cerebral microbleeds visualized with T2*-weighted MRI sequences,9 and severe diffusion and perfusion deficit.10, 11
MRI permeability imaging is a promising potential additional and distinctive marker to identify patients with an increased tendency to hemorrhagic transformation. Dedicated MRI acquisitions have been used to identify blood–brain barrier permeability derangements in patients with brain tumors for grading.12, 13 We previously reported that pretreatment permeability images derived from routine perfusion-weighted imaging source data, or slope images, may identify increased risk for hemorrhagic transformation in middle cerebral artery (MCA) stroke patients.14 However, the frequency, clinical correlates, and relation to hemorrhagic transformation of permeability derangements in the posterior circulation stroke have not been previously explored, and may differ, as ischemic volume and collateral status are different, between vertebrobasilar and MCA infarctions. The objective of this study was to characterize the frequency and relation to hemorrhagic transformation of early disruption of the blood–brain barrier, detected by MRI T2* permeability imaging, in posterior circulation ischemic patients treated with recanalization therapy.
Patients and Methods
Patient Selection
We analyzed clinical, laboratory and pretreatment MRI data on consecutive patients in a prospectively maintained database who received recanalization therapy for acute ischemic stroke within the posterior circulation at a university hospital from November 2001 to September 2009.
Imaging Methods
All patients underwent brain MRI (1.5 Tesla; Siemens Medical Systems) before recanalization therapy. The MRI protocol included diffusion-weighted imaging, gradient-recalled echo (repetition time, 800 ms; echo time 15 ms), fluid attenuated inversion-recovery sequences (repetition time, 7000 ms; echo time 105 ms, inversion time 2000 ms; matrix size, 256 x 256; field of view, 240 mm; slice thickness 5-7 mm; gap 2.5 mm), and T2* perfusion-weighted imaging sequence using MRI method previous described.15 Diffusion-weighted imaging was performed with two levels of diffusion sensitization (b=0 and 1000 sec/mm2; 5-7 mm slice thickness, no gap, and 17-20 slices). Perfusion-weighted imaging was performed with timed contrast-bolus passage technique (0.1mg/kg contrast administered into an antecubital vein with a power injector at a rate of 5cm3/sec). Perfusion-weighted imaging parameters were as follows: repetition time, 2000ms; echo time, echo time, 60 ms; 20 slices; slice thickness 7mm; no gap; matrix size, 128×96; and field of view, 240mm. Map of mean transient time > 10 seconds were generated by deconvolution of an arterial input function and tissue concentration curve.16
Derivation of Permeability Images
Permeability images were retrospectively derived from already acquired standard perfusion-weighted imaging source image data sets.14 In all tissues, T2* signal intensity normally decrease with bolus passage of gadolinium contrast through the cerebral vasculature followed by a return to baseline signal intensity. However, some tissues will then demonstrate a late, secondary decline at the terminal phase of scan acquisition. Late signal intensity decrease at the terminal phase of scan acquisition reflects accumulation of contrast within tissue parenchyma presumed to be due to blood–brain barrier disruption. In patients with blood–brain barrier compromise, gadolinium will extravasate out of vasculature and into surrounding tissue, which will result in persisting or even falling T2* intensity.14
The analysis was confined to the cases with sufficient scan acquisition time (≥ 60 seconds) to permit blood–brain barrier leakage to be discerned from analysis of late transit phases of gadolinium passage associated with contrast bolus. For each voxel, signal intensities on serial perfusion-weighted imaging source images at the terminal 10 seconds (between 50 to 60 seconds after contrast bolus) portion of the perfusion-weighted imaging acquisition were used to calculate the slope of late signal intensity change. Voxel specific rates of terminal signal intensity change were determined using linear regression technique on the ImageJ platform (ImageJ version 1.36b; National Institute of Health, Bethesda, Md). In permeability images display, voxel with signal intensity slopes over the terminal phase of scan acquisition corresponding to increasing gadolinium concentration were colored black. Background noise was subtracted from signal intensity slope images using the baseline T2* map.
Hemorrhagic Transformation Image Analysis
After recanalization therapy, all patients had a control MRI or computed tomography 24 hours after treatment. Additional imaging was obtained for any worsening in neurological status. Hemorrhagic transformation was defined and classified into 7 subtypes (modified from Berger and colleagues17): hemorrhagic infarct type 1 (HI-1), small petechiae along the margins of the infarction; hemorrhagic infarct type 2 (HI-2); more confluent petechiae within the infarction area but without the space occupying effect; parenchymal hematoma type 1 (PH-1), defined as hematoma in less than 30% of infarcted area with some space occupying effect; parenchymal hematoma type 2 (PH-2), hematoma in more than 30% or the infarcted area with substantial space-occupying effect; remote hematoma, intraparenchymal hematoma occurring outside the ischemic field; intra-ventricular hemorrhage; and subarachnoid hemorrhage. Patients could have more than one hemorrhagic type.
Two investigators who were blinded to follow-up images and clinical information independently reviewed pretreatment MRIs to determine the presence of signal changes on permeability images, based on visual inspection. Follow-up image for hemorrhagic transofrmation classification (HI1, HI2, PH1 and PH2) was reviewed by a third investigator. Pretreatment gradient-recalled echo images were also reviewed for microbleeds. The local Institutional Review Board approved the study.
Differences between the groups were examined by Fisher's exact test for categorical data and Mann-Whitney test for continuous data. For all analyses, P < 0.05 was considered statistically significant and 0.05 ≤ P < 0.20 was considered a trend.
Results
Among 21 patients with posterior circulation ischemic stroke who received recanalization therapy during the study period, pre-treatment perfusion-weighted imaging was not performed in 7 patients. The characteristics of the 14 patients meeting study entry criteria are shown in Table 1. Mean age was 71.1 years old and 5 patients (43%) were female. Median pretreatment National Institute of Health Stroke Scale (NIHSS) score was 20.5 (ranged 0-36). In the 13 patients with deficits at time of intervention, median time to recanalization therapy start was 14 hours (range 2.2-120 hours). Another one patient with basilar occlusion and repeated transient ischemic attacks was treated 2 days after resolution of the recent event. Revascularization interventions included intravenous thrombolysis only in 1, intra-arterial pharmacological thrombolysis only in 1, mechanical device only (mechanical embolus removal in cerebral ischemia [MERCI] retrieval system, wire disruption, angioplasty, and/or stent) in 8, and combination of pharmacologic thrombolysis and mechanical device in 4. None of the patients had microbleeds on pretreatment gradient-recalled echo imaging. Post-intervention angiography showed complete recanalization in 9 patients, partial recanalization in 3 patients, and no recananization in 2 patients.
Table 1.
Patient characteristics and findings
| Patient | Age/ Sex |
Admit NIHSS |
Admit blood pressure, mmHg |
Site of occlusion |
Time to intervention, h |
Intervention | Recanalization | Baseline Ischemic volume on DWI, cm3 |
Baseline FLAIR |
Baseline permeability imaging |
Hemorrhagic transformation (imaging) |
Hemorrhagic transformation (clinical) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 91/F | 15 | 160/80 | Mid BA occlusion | 12 | IA tPA 20mg | None | 0.42 | Negative | NL | No | No |
| 2 | 78/M | 36 | 160/90 | Distal BA occlusion | 20 | MERCI | Complete | 0.32 | Positive | NL | No | No |
| 3 | 86/M | 24 | 137/58 | Distal BA occlusion | 8.5 | MERCI and IA tPA 6mg | Complete | 4.65 | Negative | NL | HI-1 at left thalamus | Asymptomatic |
| 4 | 70/M | 26 | 170/90 | Mid BA occlusion | 22 | IA tPA 2.2mg and ReoPro 23mg and angioplasty | Complete | 3.07 | Negative | NL | HI-2 at bilateral pons | Asymptomatic |
| 5 | 85/F | 20 | 180/120 | Left proximal PCA occlusion | 7 | Catheter and wire disruption | Complete | 2.61 | Negative | NL | No | No |
| 6 | 68/M | 35 | 190/110 | Proximal right VA, distal BA, and left proximal PCA occlusion | 10 | IA tPA 20mg and MERCI | Partial | 1.72 | Negative | NL | HI-2 at right thalamus | Asymptomatic |
| 7 | 56/F | 21 | 220/103 | Proximal and mid BA occlusion | 17 | MERCI | Partial | 9.04 | Positive | NL | No | No |
| 8 | 50/M | 33 | 221/96 | Left distal VA to mid BA occlusion | 8 | MERCI | None | 16.7 | Negative | NL | No | No |
| 9 | 67/M | 4 | 134/86 | Proximal BA occlusion | 28 | Angioplasty and stent | Complete | 0.55 | Positive | NL | IVH | Asymptomatic |
| 10 | 79/M | 0 | 157/80 | Mid BA tight stenosis | Repeat VBI 15 days before intervention | Angioplasy and stent | Complete | 0 | Negative | NL | No | No |
| 11 | 68/F | 1 | 135/88 | Mid to distal BA occlusion | 2.2 | IV tPA 60mg | Complete | 0 | Negative | NL | No | No |
| 12 | 62/M | 8 | 71/43 | Right VB junction tight stenosis | 120 | Angioplasty and stent | Partial | 24.7 | Positive | NL | No | No |
| 13 | 63/F | 23 | 212/79 | Mid BA tight stenosis | 4 | Angioplasty and stent | Complete | 0.60 | Negative | NL | No | No |
| 14 | 73/F | 8 | 136/90 | Mid BA occlusion | 14 | IA tPA 7mg and angioplasty | Complete | 1.56 | Positive | Abnormal | PH-2 at pons and midbrain | Symptomatic |
BA: basilar artery; F: female; HI-1: hemorrhagic infarct type 1; HI-2: hemorrhagic infarct type 1; IA: intra-arterial; IV: intravenous; IVH: intraventricular hemorrhage; M: male; MERCI: mechanical embolus removal in cerebral ischemia; MRA: magnetic resonance angiography; NIHSS: National Institute of Health Stroke Scale; NL: normal; PH-1: parenchyma hematoma type 1; PH-2: parenchyma hematoma type 2; VA: vertebral artery; VBI: vertebrobasilar insufficiency
Among all 14 patients, post-treatment parenchymal hematoma (PH-2) occurred in 1 and more minor degrees of hemorrhagic transformation in 4 (HI-2 in 2, HI-1 in 1, and intra-ventricular hemorrhage in 1). Patients with and without hemorrhagic transformation did not differ in age, NIHSS score, diastolic or systolic blood pressure, blood glucose, total cholesterol, platelet count, activated partial thromboplastin time, and rate of atrial fibrillation. However, all the patients who received combination of pharmacological thrombolysis and mechanical device had hemorrhagic transformation (Likelihood ratio 11.7, Fisher's exact test p=0.002).
Pretreatment permeability imaging abnormality was present in 1 of the 14 (7%) posterior circulation stroke patients. The one patient with pretreatment permeability abnormality was the one patient to develop post-treatment parenchymal hematoma (100% vs. 0%, Fisher's exact test, P=0.07) (Figure 1). In four patients with other types of hemorrhagic transformation, pretreatment imaging did not show an increased permeability abnormality.
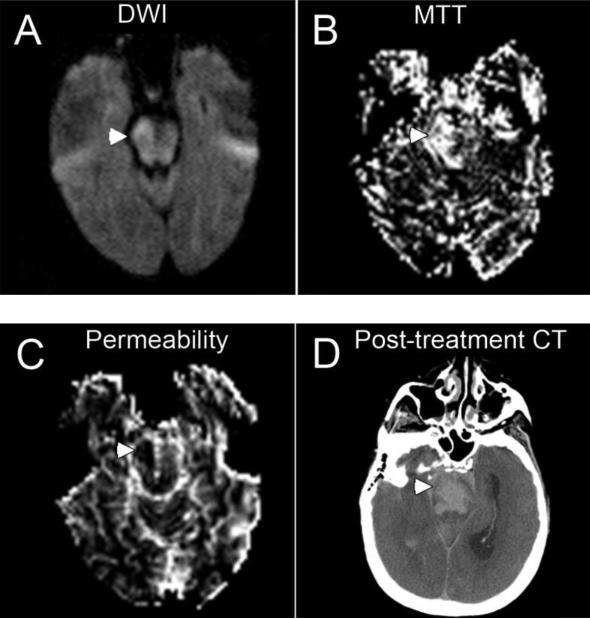
Figure 1.
Pretreatment magnetic resonance imaging and post-treatment computed tomographic (CT) findings in a patient (patient 14) with parenchymal hematoma. Diffusion sequence shows right pontine infarct (arrowhead) (A); perfusion sequence demonstrates delayed mean transient time in right pons (arrowhead) (B); and permeability image shows abnormality hypointensity in right pons (arrowhead) (C). The area of hypodensity on this permeability-image indicates tissue with breakdown of the blood-brain barrier and consequent contrast-staining of the parenchyma on late T2* images following bolus administration. Post-treatment CT show pontine hematoma and subarachnoid hemorrhage (arrowhead) (D).
Discussion
This study is the first to characterize the frequency of pretreatment permeability abnormalities in large artery posterior circulation ischemic stroke and their relation to hemorrhagic transformation. We found permeability derangement in 7% of patients, and the patient with this abnormality was the only patient to evolve a major parenchymal hematoma after recanalization therapy.
Most investigations of hemorrhagic transformation after recanalization therapy to date have focused on anterior circulation infarcts4, 7, 14 and there is little data available in posterior circulation stroke. Compared to anterior circulation occlusions, vertebral or basilar artery occlusions typically cause smaller ischemic lesion volumes, which may reduce the parenchymal hematoma rate. Clinical and laboratory features predictive of hemorrhagic transformation in the anterior circulation, such as age, NIHSS score, blood pressure, and serum glucose, did not predict hemorrhagic transformation in posterior circulation ischemic stroke in this series. In the only patient to develop parenchymal hematoma, pretreatment clinical presentation and laboratory data were not suggestive of hemorrhagic transformation risk, with blood pressure 136/90 mmHg, NIHSS score 8, blood glucose 99 mg/dL, cholesterol 194 mg/dL, platelet 198*103/μl, and no microbleeds. However, pretreatment permeability imaging showed abnormality of the blood–brain barrier had already developed, and the patient evolved a substantial pontine hematoma. In the anterior circulation, indices of blood–brain barrier disruption, including pretreatment permeability imaging and serum metalloproteinase-9 levels, have shown to be associated with parenchymal hematoma after recanalization therapy.14, 18 The current study extends this finding to the posterior circulation. Since traditional clinical and laboratory predictors of hemorrhagic transformation are less powerful in the posterior circulation, this imaging biomarker of hematoma risk may be particularly useful in acute vertebral and basilar occlusions. Our data suggest permeability imaging can be a possible predictor in posterior circulation infarction to identify individuals with blood–brain barrier disruption who are at high risk for harmful effects of treatment because of parenchymal hematoma.
While permeability abnormality predicted major hematoma, it did not foretell minor petechial hemorrhages after recanalization therapy in this series. This finding accords with a similar observation in anterior circulation infarcts.14 Two potential explanations for this observation can be considered, one biological and one technological. The biological consideration is that some patients who develop petechial hemorrhages had fully intact blood–brain barrier at the time of MRI, but developed some blood–brain barrier disruption and loss of vessel wall integrity later in the course. The technical consideration is that the pragmatic permeability imaging technique in this study may be insensitive to very mild blood–brain barrier disruptions. We performed permeability analyses on standard perfusion MRI acquisitions. Dedicated perfusion acquisitions likely are more sensitive, but currently require additional contrast exposure and more prolonged acquisition times, so are difficult to incorporate into acute stroke imaging. However, compared with absence of hemorrhagic transformation, only parenchymal hematoma type 2 is associated with an increased risk for neurological deterioration after ischemic stroke with thrombolysis therapy.17, 19 Therefore deriving permeability imaging from standard perfusion acquisitions is likely to be a useful tool to identify patients at risk of hemorrhagic transformations that would worsen final outcome. We found patients receiving combinations of multiple recanalization interventions, including pharmacological thrombolysis and mechanical devices, had a higher rate of hemorrhagic transformation, including parenchymal hematoma. This observation is consonant with the experience in MCA infarction series.14 It is likely that this association results both from more prolonged ischemia due to refractory occlusions, which prompted combined therapy, and from injury to the vessel wall due to repeated instrumentation and trespass in the course of combined therapy. In the future, pretreatment permeability imaging abnormality may identify patients in whom a more conservative approach to treatment should be pursued.
There are several limitations in our study. The small sample size constrains the precision of statistical results and the current study is essentially an observation of an association between a single post-treatment parenchymal hematoma event and a baseline perfusion-weighted imaging characteristic in a small series of posterior circulation ischemic stroke. Also, patients were treated with a variety of recanalization therapies, including pharmacological thrombolysis alone (intravenous or intra-arterial), mechanical device alone, or combined therapy. These different treatment modalities are likely to have both commonalities and contrasts in the features that predict hemorrhagic transformation after their use. Larger, multicenter studies are warranted to confirm and refine the role of permeability imaging in predicting hemorrhagic transformation after recanalization therapy in posterior circulation stroke.
In conclusion, pretreatment permeability abnormality, an indicator of blood–brain barrier derangements, is an infrequent finding in acute posterior circulation ischemic stroke and may be associated with an increased risk of parenchymal hematoma development undergoing recanalization therapy. With confirmation and refinement in larger studies, permeability imaging may enhance the benefit-risk ratio of recanalization therapy in posterior circulation stroke patients.
Acknowledgements
Meng Lee (CMRPG 660311, Taiwan), Jeffrey L Saver and Jeffry Alger (NIH SPOTRIAS), and David S Liebeskind (NIH-NINDS Awards K23NS054084 and P50NS044378), Bruce Ovbiagele (UCLA-RCMAR under NIH/NIA Grant P30-AG021684).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: JLS and DSL designed the study. ML, JLS, QH, SS, LKA, DK, BO, PMV, MTF, MST, NS, JPV, RJ, GRD, ST, NG, FV participated in the data collection and extraction. ML processed the perfusion imaging with guidance from JRA and DSL. ML did the statistical analysis with guidance from JLS and DSL. ML wrote the first draft of the report, and JLS and DSL did the major revision. All other authors commented on the draft and approved the final version.
Conflicts of Interest: None
Disclosures: None
Reference
- 1.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 2.Lansberg MG, Thijs VN, Bammer R, Kemp S, Wijman CA, Marks MP, Albers GW. Risk factors of symptomatic intracerebral hemorrhage after tPA therapy for acute stroke. Stroke. 2007;38:2275–8. doi: 10.1161/STROKEAHA.106.480475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The IMS Study Investigators Hemorrhage in the Interventional Management of Stroke study. Stroke. 2006;37:847–51. doi: 10.1161/01.STR.0000202586.69525.ae. [DOI] [PubMed] [Google Scholar]
- 4.Kase CS, Furlan AJ, Wechsler LR, Higashida RT, Rowley HA, Hart RG, Molinari GF, Frederick LS, Roberts HC, Gebel JM, Sila CA, Schulz GA, Roberts RS, Gent M. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology. 2001;57:1603–10. doi: 10.1212/wnl.57.9.1603. [DOI] [PubMed] [Google Scholar]
- 5.Kidwell CS, Saver JL, Carneado J, Sayre J, Starkman S, Duckwiler G, Gobin YP, Jahan R, Vespa P, Villablanca JP, Liebeskind DS, Vinuela F. Predictors of hemorrhagic transformation in patients receiving intra-arterial thrombolysis. Stroke. 2002;33:717–24. doi: 10.1161/hs0302.104110. [DOI] [PubMed] [Google Scholar]
- 6.Kohrmann M, Juttler E, Fiebach JB, Huttner HB, Siebert S, Schwark C, Ringleb PA, Schellinger PD, Hacke W. MRI versus CT-based thrombolysis treatment within and beyond the 3 h time window after stroke onset: a cohort study. Lancet Neurol. 2006;5:661–7. doi: 10.1016/S1474-4422(06)70499-9. [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–17. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 8.Neumann-Haefelin T, Hoelig S, Berkefeld J, Fiehler J, Gass A, Humpich M, Kastrup A, Kucinski T, Lecei O, Liebeskind DS, Rother J, Rosso C, Samson Y, Saver JL, Yan B. Leukoaraiosis is a risk factor for symptomatic intracerebral hemorrhage after thrombolysis for acute stroke. Stroke. 2006;37:2463–6. doi: 10.1161/01.STR.0000239321.53203.ea. [DOI] [PubMed] [Google Scholar]
- 9.Kidwell CS, Saver JL, Villablanca JP, Duckwiler G, Fredieu A, Gough K, Leary MC, Starkman S, Gobin YP, Jahan R, Vespa P, Liebeskind DS, Alger JR, Vinuela F. Magnetic resonance imaging detection of microbleeds before thrombolysis: an emerging application. Stroke. 2002;33:95–8. doi: 10.1161/hs0102.101792. [DOI] [PubMed] [Google Scholar]
- 10.Alsop DC, Makovetskaya E, Kumar S, Selim M, Schlaug G. Markedly reduced apparent blood volume on bolus contrast magnetic resonance imaging as a predictor of hemorrhage after thrombolytic therapy for acute ischemic stroke. Stroke. 2005;36:746–50. doi: 10.1161/01.STR.0000158913.91058.93. [DOI] [PubMed] [Google Scholar]
- 11.Selim M, Fink JN, Kumar S, Caplan LR, Horkan C, Chen Y, Linfante I, Schlaug G. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke. 2002;33:2047–52. doi: 10.1161/01.str.0000023577.65990.4e. [DOI] [PubMed] [Google Scholar]
- 12.Roberts HC, Roberts TP, Bollen AW, Ley S, Brasch RC, Dillon WP. Correlation of microvascular permeability derived from dynamic contrast-enhanced MR imaging with histologic grade and tumor labeling index: a study in human brain tumors. Acad Radiol. 2001;8:384–91. doi: 10.1016/S1076-6332(03)80545-7. [DOI] [PubMed] [Google Scholar]
- 13.Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24:1989–98. [PMC free article] [PubMed] [Google Scholar]
- 14.Bang OY, Buck BH, Saver JL, Alger JR, Yoon SR, Starkman S, Ovbiagele B, Kim D, Ali LK, Sanossian N, Jahan R, Duckwiler GR, Vinuela F, Salamon N, Villablanca JP, Liebeskind DS. Prediction of hemorrhagic transformation after recanalization therapy using T2*-permeability magnetic resonance imaging. Ann Neurol. 2007;62:170–6. doi: 10.1002/ana.21174. [DOI] [PubMed] [Google Scholar]
- 15.Shih LC, Saver JL, Alger JR, Starkman S, Leary MC, Vinuela F, Duckwiler G, Gobin YP, Jahan R, Villablanca JP, Vespa PM, Kidwell CS. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke. 2003;34:1425–30. doi: 10.1161/01.STR.0000072998.70087.E9. [DOI] [PubMed] [Google Scholar]
- 16.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–25. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 17.Berger C, Fiorelli M, Steiner T, Schabitz WR, Bozzao L, Bluhmki E, Hacke W, von Kummer R. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001;32:1330–5. doi: 10.1161/01.str.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 18.Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, Quintana M, Alvarez-Sabin J. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- 19.Saver JL. Hemorrhage after thrombolytic therapy for stroke: the clinically relevant number needed to harm. Stroke. 2007;38:2279–83. doi: 10.1161/STROKEAHA.107.487009. [DOI] [PubMed] [Google Scholar]