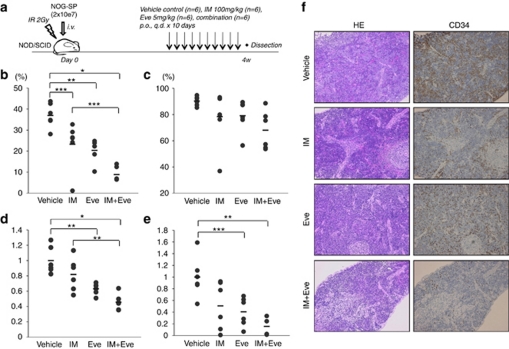
Figure 4.
In vivo effects of everolimus on leukemic spleen cells in combination with imatinib. (a) Non-obese diabetic/severe combined immunodeficiency ( mice were irradiated (IR), and leukemic spleen (SP) cells from NOG (2 × 107) were injected. Experiments were performed twice with three in each treatment group. Control vehicle (n=6), imatinib 100 mg/kg (n=6), everolimus 5 mg/kg (n=6) or a combination of both (n=6) were administered for 10 days, and mice were dissected 24 h after the last administration on day 28 following the tumor injection. (b, c) Percentages of CD19+ cells in peripheral blood (b) and bone marrow (c) are shown, respectively. (d) Spleen weight was relatively compared with the average of control mice in each experiment. Bars indicate average of spleen weight in each study group. (e) CD19+ leukemic spleen cell numbers were relatively compared with the average of control (b–e: *P<0.001, **P<0.005 and ***P<0.05 by one-way analysis of variance followed by Bonferroni). (f) Hematoxylin and eosin (HE) staining (left panels) and immunohistochemical analysis with CD34 (right panels) of the spleen from control mice and imatinib and/or everolimus-treated mice were performed. A charge-coupled device camera provided images at approximately × 100 of the original magnification.