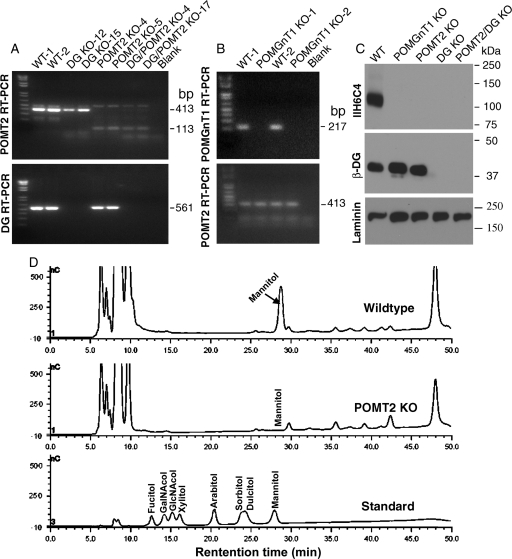
Fig. 1.
Characterization of POMGnT1, DG, POMT2 and DG/POMT2 double-KO neural stem cells. RT–PCR (A and B) and western blot analysis (C) were carried out to confirm the loss of DG, POMGnT1 and POMT2 expression in respective clones. Mannitol analysis was carried out by HPAEC-PAD on proteins isolated from wild-type and POMT2 KO neural stem cells after reductive β-elimination (D). (A) RT–PCR with DG or POMT2 primers. For POMT2, the 413 bp amplicon expected for wild-type mRNA was only observed for wild-type and DG KO clones. In contrast, the 113 bp amplicon expected for the KO mRNA was only observed for POMT2 KO and double-KO clones. For DG, the 561 bp amplicon expected for the wild-type mRNA was detected only in wild-type and POMT2 KO cells but not in DG KO and double-KO cells. (B) POMGnT1 RT–PCR. The 217 bp amplicon expected for the wild-type mRNA was present in wild-type but not in POMGnT1 KO cells. (C) Western blot analysis with IIH6C4, β-DG and laminin antibodies. IIH6C4 immunoreactivity was detected at 120 kDa in wild-type neural stem cells, but not in any other KO cell lines. β-DG immunoreactivity was detected only in wild-type, POMGnT1 KO and POMT2 KO neural stem cells, but not in DG KO or DG/POMT2 double-KO cells. As a loading control, laminin expressed by neural stem cells was readily detected in all cells. (D) Mannitol analysis. Mannitol peak was present in wild-type neural stem cells but only background was observed for POMT2 KO neural stem cells.