SUMMARY
The causes of post-restriction hyperphagia (PRH) represent a target for drug-based therapies to prevent obesity. However, the factors causing PRH are poorly understood. We show that, in mice, the extent of PRH was independent of the time under restriction, but depended on its severity, suggesting that PRH was driven by signals from altered body composition. Signals related to fat mass were important drivers. Circulating levels of leptin and TNFα were significantly depleted following caloric restriction (CR). We experimentally repleted their levels to match those of controls, and found that in both treatment groups the level of PRH was significantly blunted. These data establish a role for TNFα and leptin in the non-pathological regulation of energy homeostasis. Signals from adipose tissue, including but not limited to leptin and TNFα, regulate PRH and might be targets for therapies that support people engaged in CR to reduce obesity.
INTRODUCTION
Levels of obesity in the western world have expanded progressively since the early 1960s; currently, over 20% of the population in many western countries is obese, with a further 30–40% overweight (Andersen et al., 2005; Berg et al., 2005; Czernichow et al., 2009; Flegal et al., 2002; Flegal et al., 2004; Flegal et al., 2010). This epidemic has spread to developing countries and achieved a widespread global importance (Abubakari et al., 2008; Kain et al., 2002; Raj et al., 2007). Obesity is a large risk factor for several serious clinical disorders, including diabetes, hypertension, non-alcoholic fatty liver disease and cancer (Allison et al., 2002; Baldelli et al., 2008; Bramlage, 2008; Nock et al., 2008; Osorio-Costa et al., 2009; Sanyal, 2011; Zhu et al., 2002), which together lead annually to billions of dollars of healthcare spend (Finkelstein et al., 2009; Tsai et al., 2011; Wolf and Colditz, 1998) and excess mortality (Flegal and Graubard, 2009; Flegal et al., 2007a; Flegal et al., 2007b).
The most frequent self- and physician-prescribed treatment for obesity is caloric restriction (CR) or dieting. Among adolescents, up to 30% of individuals report being frequent or infrequent users of CR to control body weight (Field et al., 2001), and 62% of female and 29% of male high school students reported trying to lose weight in the United States in 2005 (Lowry et al., 2005). CR is attractive because it yields immediate positive results in terms of reducing both body and fat mass (Astrup et al., 2000; Bradley et al., 2009; Das et al., 2009). CR, however, is often unsuccessful for long-term obesity treatment (Aronne et al., 2009; Mark, 2008). This is in part because patients engaged in CR develop compensatory mechanisms to oppose the energy imbalance combined with a strong hyperphagic drive (Doucet and Cameron, 2007; Dulloo et al., 1996; Dulloo et al., 1997; Leibel et al., 1995; Ravussin et al., 2011; Rosenbaum et al., 2002; Rosenbaum et al., 2003; Rosenbaum et al., 2005; Rosenbaum et al., 2008). This leads them to cheat on their diets (Del Corral et al., 2011), terminate CR and ultimately regain any lost weight and fat: often called obesity rebound or catch-up fat (Crescenzo et al., 2003; Dulloo et al., 2006; Mann et al., 2007; Yepuri et al., 2011). In animal models of this phenomenon, the strong hyperphagic drive conditioned by CR is reflected in a profound hyperphagia and weight regain when animals are released from CR back onto ad libitum (AL) food: post-restriction hyperphagia (PRH) (Hambly and Speakman, 2005; MacLean, 2005; MacLean et al., 2004; MacLean et al., 2006). Understanding PRH is important because it represents a potentially useful target for pharmaceutical-based interventions that will support the popular CR approach.
Despite its importance, our understanding of PRH and weight regain is surprisingly poor (Crujeiras et al., 2010; Jackman et al., 2008; Labayen et al., 2011; MacLean, 2005; MacLean et al., 2006; MacLean et al., 2011). In particular, we do not know whether the hyperphagic drive stems directly from the experience of negative energy balance, or whether altered body composition is the primary driver. This is because these two factors are normally closely correlated. In the field of reproductive biology, it has been widely assumed that reproductive function depends on body composition (Frisch, 1993; Frisch, 1994; Frisch, 1996). In fact, function depends on immediate experience of energy balance (Schneider, 2004; Schneider et al., 2000; Wade and Schneider, 1992), which makes it clear that we cannot automatically assume that the driver is related to loss of body tissue. If altered body composition does contribute to PRH it is uncertain whether reductions in fat mass (FM) or fat-free mass (FFM) are of key significance (Crescenzo et al., 2011; Dulloo et al., 1997; Dulloo and Jacquet, 1998). Moreover, the signals that derive from these tissues that stimulate PRH remain obscure. A role for reduced leptin has been previously demonstrated in the adaptations to CR, including some of the post-restriction responses and weight regain (Rosenbaum et al., 2002; Rosenbaum et al., 2005). In this paper we show that PRH in mice is caused by altered body composition rather than the experience of energy imbalance. We show that reduced FM rather than altered FFM is the key factor driving PRH, and that the main signals from FM that drive PRH include reduced levels of tumour necrosis factor alpha (TNFα) and leptin. These data establish a role for TNFα in the non-pathological regulation of energy homeostasis.
RESULTS
Experiment one: effect of restriction period on PRH
In order to determine whether the extent of PRH was related to the length of time that a subject experienced CR, we exposed mice to either 25 or 75 days of restricted food rations. Average body mass (BM) loss was kept consistent within the two groups to ensure that the only difference was the length of time that they experienced a restricted diet. Prior to dietary restriction there was no significant difference in BM, FM, FFM or food intake (FI) between any of the groups (ANOVA: P>0.3). Absolute FIs of the restricted mice were 3.7±0.08 g/day for the short-term restricted group and 3.8±0.09 g/day for the long-term restriction group. Over the measurement period, control mice gradually increased BM at a rate of 0.06 g/day so that, by the end of the study, it was significantly elevated (paired t-test: t=4.67, P=0.003). These changes consisted mainly of increases in FM by 2.8 g (paired t-test: t=6.16, P=0.001), but also of significant increases in FFM by 0.2 g (paired t-test: t=2.53, P=0.045). Daily FI, however, declined significantly over the measurement period by 1.1 g (paired t-test: t=5.39, P=0.002).
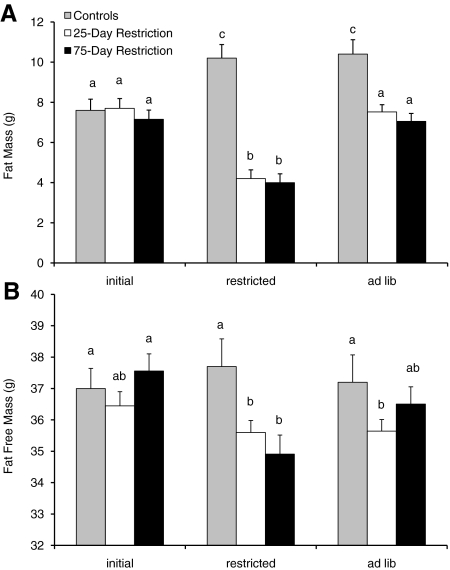
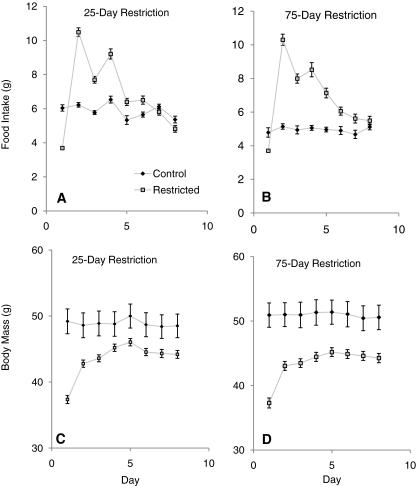
The short-term restricted mice lost on average 9.2±0.50 g over the 25 days of dietary restriction and showed rapid weight loss over the first 15 days but then a more gradual rate of loss approaching a plateau for the last 10 days. This loss consisted of significant reductions in both FM (by 3.5±0.35 g; paired t-test: t=9.94, P<0.001) and FFM (by 0.84±0.26 g; paired t-test: t=3.25, P=0.003) (Fig. 1; supplementary material Table S1). The mice on long-term restriction did not change BM significantly between 25 and 75 days, and over the whole restriction period lost on average 9.6±0.64 g. This loss consisted of significant reductions in both FM (by 3.2±0.17 g; paired t-test: t=9.94, P<0.001) and FFM (by 2.61±0.31 g; paired t-test: t=3.25, P=0.003) (Fig. 1). The discrepancy between BM loss and combined FM and FFM loss was probably due to differences in gut fill because restricted mice were measured prior to feeding when the gut was empty. After release from both long-and short-term restriction there were almost identical patterns of hyperphagia (Fig. 2A,B). On the first day of release, the short-term restricted mice consumed 10.5 g, whereas the long-term restricted mice consumed 10.4 g. This indicates that the hyperphagic drive was equally strong in both groups and that the extra 50 days spent under restriction had no effect on their hunger. Over the days post-restriction, the hyperphagia gradually subsided so that, by the final day of measurement, 7 days after release from restriction, the FI of either group was not significantly different from that of the controls or each other (ANOVA: P>0.05). The accumulated FI over the 7 days of AL feeding was almost identical in the two restricted groups (short-term restricted FI: 50.9±1.24 g; long-term restricted FI: 51.4±1.19 g; ANOVA: F1,56=0.05, P=0.83) and both were significantly higher than the controls (FI=40.3±0.8 g over the same time period; ANOVA: short-term controls, F1,39=20.00, P<0.001; long-term controls, F1,31=19.66, P<0.001).
Fig. 1.
Body composition changes throughout experiment one. (A,B) FM and FFM changes before, during and after either a 25-day or 75-day restriction. Control data are shown for the corresponding time periods. Columns with the same letter are not significantly different (one-way ANOVA). Data are shown ± s.e.m. See also supplementary material Table S1.
Fig. 2.
Recovery after short- and long-term dietary restriction. (A–D) FI and BM for the two restriction periods after release from the dietary restriction (25-day short-term restriction or 75-day long-term restriction). Data are shown in comparison to age-matched controls. For both restricted groups of mice there was a marked hyperphagia after release from the restricted diet and rapid BM increase. Data are shown ± s.e.m. See also supplementary material Table S1.
On release from the restriction, BM in both groups increased significantly over the first 24 hours (by 5.4 g for the short-term restricted group and by 5.7 g for the long-term restricted group; paired t-test: P<0.05; Fig. 2C,D). This was probably mostly due to increased gut fill. The increase in BM was not significantly different between the two groups (ANOVA: F1,56=0.18, P=0.19). This rapid increase was followed by a more gradual but significant increase in BM in both groups (paired t-test: P<0.05). On the final day of measurement there was no significant difference in BM between the mice of the two restriction groups, with a mean BM of 44.2 g for the short-term group and 44.3 g for the long-term group (ANOVA: F1,56=0.01, P=0.94), but this was significantly lower than the controls that were measured at the same time point (ANOVA: short-term controls, F1,39=0.88, P=0.005; long-term controls, F1,33=12.57, P=0.001).
When allowed ad libitum access to food after dietary restriction, the short-term restricted mice gained 3.3±0.18 g of fat, whereas the long-term restricted group gained 3.1±0.25 g of fat, which was significantly greater than both of their levels during the restriction period (paired t-test: short-term restricted, t=13.39, P<0.001; long-term restricted, t=12.05, P<0.001) (Fig. 1). These increases were not significantly different between the two groups (ANOVA: F1,56=0.63, P=0.43). The FM after AL feeding was not significantly different from the pre-restriction level in the same individuals (paired t-test: short-term restricted, t=0.59, P=0.56; long-term restricted, t=0.27, P=0.79); however, it was significantly lower than the FM of the control animals (ANOVA: short-term restricted, F1,39=7.26, P=0.01; long-term restricted, F1,33=19.51, P<0.001). On release from restriction there was, however, a significant increase in FFM from that measured at the end of restriction in the long-term restricted group only (paired t-test: t=5.17, P<0.001) and not in the short-term restricted group (paired t-test: t=0.78, P=0.44). The FFM after AL feeding was not significantly different to the initial pre-restriction level in the same mice for both groups (paired t-tests: P>0.05).
Experiment two: do signals from FM or FFM drive the PRH?
The data presented above suggest that, over prolonged periods of CR, subjects neither get accustomed to the energy deficit that causes a reduction in their experience of hunger, nor do they accumulate a greater hunger drive with long periods of CR. This indicates that signals from FM or FFM might be the driving factor behind PRH. In this second experiment, we determined whether the degree of CR affected PRH by placing mice on two different levels of restriction (65% or 50% of their measured daily intake). FM and FFM were monitored throughout, and we also measured the levels of potential key circulating hormones and neuropeptides, both before and after the animals had received their daily ration.
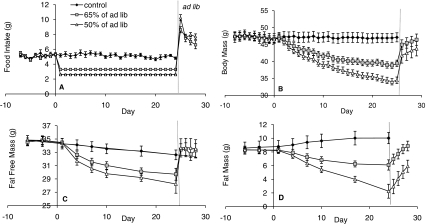
There were no significant differences in FI, BM, FM or FFM between the groups prior to restriction (P>0.05). FI averaged 5.11±0.09 g/day over all 68 mice, which decreased slightly, but significantly, during the remainder of the study for the control group with AL feeding (mean on final day=4.93±0.20 g/day; paired t-test: t=2.02, P=0.05). The group restricted to 65% of their ad lib FI (low restriction group) were restricted to an average of 3.3±0.09 g/day, whereas the 50% restricted group (high restriction) were restricted to an average of 2.6±0.07 g/day (Fig. 3A; supplementary material Table S2). On release from restriction, there was a large hyperphagia in both groups, with FI reaching 8.6±0.41 g/day in the low restriction group and being significantly greater in the high restriction group at 10.2±0.41 g on the first day after release (ANOVA: F1,16=7.63, P=0.014). Both of these values were significantly greater than the FI of the controls and remained so for the 4 days of measurement, although after the first day there was no significant difference between the two restriction groups: FI had decreased to 6.61±0.54 g/day and 7.70±0.37 g/day for low and high restriction groups, respectively (P>0.05).
Fig. 3.
Effect of high- and low-level restriction on FI and body composition. (A,B) FI and BM changes during low (to 65% of AL feeding) and high (to 50% of AL feeding) levels of restriction. For both restricted groups of mice there was a marked hyperphagia after release from the restricted diet, and this was significantly greater with increased restriction. There was also a rapid BM increase in both groups. (C,D) Changes in FFM and FM during restriction and release onto AL feeding. FFM had recovered in the first day of AL, whereas FM had a more gradual increase. Data are shown ± s.e.m. See also supplementary material Fig. S1 and Table S2.
Baseline BM averaged 47.17±1.49 g. Similar to the FI data, there was very little difference in this value in the control animals throughout the study, with an average of 47.13±1.3 g on the final day (Fig. 3B). The mice placed on restriction started losing weight by the second day, and the low restriction group lost 15% of their BM over the 25 days, whereas the high restriction group lost 26%. Significant differences in BM between the controls and the high restriction group became apparent after 4 days of restriction, whereas it took until day 7 in the low restriction group. Differences in BM appeared between the two restricted groups after day 13 of restriction and remained for the rest of the restriction period. After release from restriction, BM increased by 4.6 g (from 39.4±0.9 g to 45.0±1.4 g) in the low restriction group and 7.4 g (from 34.4±1.0 g to 41.8±2.3 g) in the high restriction group on the first day of free feeding. Some of this BM increase was caused by increased gut fill (we estimated this to be around 40% in a separate group of animals dissected before and after restriction). After 1 day of access to food, there was already no significant difference in BM between either of the restriction levels and the controls, although the BM of both restriction groups continued to rise for the remainder of the experiment and on the final day was 46.44±1.66 g for the low and 43.78±2.15 g for the high restriction group.
In the baseline measurement period, mice had on average 17.4±0.5% body fat, and FM was not correlated with FI (supplementary material Fig. S1A), unlike FFM, which was positively correlated with FI (supplementary material Fig. S1B; regression: F1,67=44.7, P<0.001). Body composition measurements followed a similar pattern to those of BM, with decreases in both FM and FFM during restriction (Fig. 3C,D). The controls significantly gained 15% (1.4 g) in FM, whereas the low restriction group lost 26% (2.2 g) and the high restriction group lost 73% (6.2 g; paired t-test: P<0.05). FM was significantly correlated with FI in the restricted mice on the last day of restriction (supplementary material Fig. S1C; regression: F1,57=46.97, P<0.001). Much smaller proportions of FFM were lost when diet was not restricted, with control mice significantly losing 6% (2.0 g) of FFM, whereas the low restriction group lost 14% (5.2 g) and the high restriction group lost 19% (6.6 g; paired t-test: P<0.05). FFM was also significantly correlated with FI in restricted mice (supplementary material Fig. S1D; regression: F1,57=52.41, P<0.001). With both FM and FFM, differences between controls and the restricted groups became apparent after day 7, whereas the restricted groups only separated from each other around day 17 for FM and did not differ over the restriction period for FFM. When mice were released from restriction, FFM immediately increased significantly to the level of the controls in both restricted groups and remained stable at that level for the 4 days of free access to food. This amounted to an increase in FFM of 3.72±0.28 g in the low restriction group and 5.42±0.34 g in the high restriction group in 1 day, which was not significantly different from the final control measurement (ANOVA Tukey pairwise comparison: F2,25=0.20, P=0.82). During the AL phase, FFM was also significantly correlated with the total mass of food consumed over the 4 days of AL feeding (supplementary material Fig. S1F; regression: F1,17=7.02, P=0.02). The diet contained 20.9% protein and, for the high group, if all the available protein was devoted to increasing lean tissue mass, this would require 5.8 g of food to be eaten to accumulate 5.42 g of wet lean tissue, assuming that lean tissue is 72% water. The actual FI was 10.24 g in the high restriction group and therefore they ate far more protein than was observed as increased FFM. FM followed a much more gradual increase over the 4 days. The low restriction group gained on average 0.69 g per day, whereas the high restriction group gained 0.91 g per day. By the final day of study, the low restriction group had increased their FM to the same levels as the controls, whereas the high restriction group still had a significantly lower FM (ANOVA Tukey pairwise comparisons: F2,25=0.69, P=0.004). FM during the AL phase was not correlated with FI as was observed during baseline (supplementary material Fig. S1E).
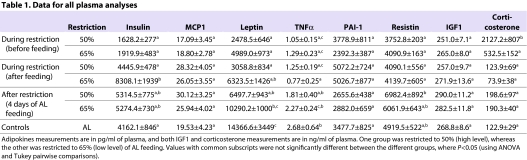
Analysis of plasma revealed that circulating levels of insulin-like growth factor (IGF1), monocyte chemotactic protein 1 (MCP1; also known as C-C motif chemokine 2) and plasminogen activator inhibitor 1 (PAI-1) were not significantly different between groups for different levels of restriction or at either time point (before or after mice received their daily ration) (Table 1). The levels of resistin were significantly higher in the high restriction group only, during the release to the AL feeding phase. However, during the restriction period, there were no significant differences in resistin from the controls. There was no difference between the groups for insulin, except after feeding in the low restriction group, which had a highly elevated insulin concentration. Only in the high restriction group and only prior to feeding was the level of circulating corticosterone significantly higher, compared with all other groups. The levels rapidly decreased after food was provided.
Table 1.
Data for all plasma analyses
The mice on restriction, regardless of whether the samples were collected before or after feeding, had lower circulating levels of TNFα and leptin than the controls, and these levels were correlated with FI in the restricted animals (regression: leptin, F1,38=23.75, P<0.001; TNFα, F1,31=5.16, P=0.03). These levels increased again when mice were provided with AL food for 4 days, so that the levels approached or were not significantly different from the controls depending on which level of restriction the mice were under. The fact that the levels of these two adipokines were low during restriction and that the hyperphagia still existed after 4 days while FM was still recovering, unlike FFM, suggested that signals from FM were most likely to be driving the hyperphagia. Leptin and TNFα, both secreted by fat tissue, are strong candidates for involvement in the process.
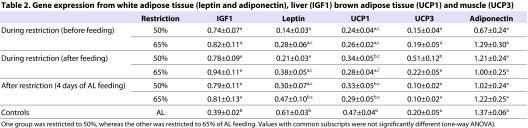
In addition, several tissues were analysed and the details are shown in Table 2. Unlike circulating levels of IGF1, there was a significant difference in liver IGF1 expression between the groups, with expression significantly lower in the controls. Leptin in white adipose tissue (WAT) and uncoupling protein 1 (UCP1) expression in brown adipose tissue (BAT) showed the opposite pattern, with controls having the highest expression level. A positive relationship was observed with FI in the restricted mice for leptin only (regression: F1,37=6.52, P=0.015). There were no differences in the expression of UCP3 in muscle or adiponectin in WAT between the groups.
Table 2.
Gene expression from white adipose tissue (leptin and adiponectin), liver (IGF1) brown adipose tissue (UCP1) and muscle (UCP3)
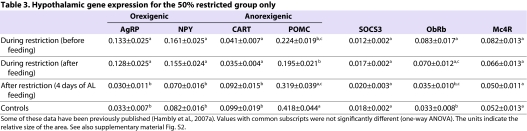
Expressions of the orexigenic neuropeptides agouti related peptide (AgRP) and neuropeptide-Y (NPY) were significantly increased in the arcuate nucleus (ARC) of the hypothalamus of restricted mice both before and after feeding, compared with controls and mice that had been released from restriction for 4 days [Table 3; some of these data previously published by Hambly et al. (Hambly et al., 2007a)]. The anorexigenic neuropeptides pro-opiomelanocortin (POMC) and cocaine and amphetamine regulated transcript (CART) showed the opposite pattern in expression. In addition, there were differences in leptin receptor (ObRb) levels between the controls and restricted mice only (not between the restricted groups). Levels of neither suppressor of cytokine signalling 3 (SOCS3) or melanocortin 4 receptor (MC4R) [in the paraventricular nucleus (PVN)] showed any differences between the groups.
Table 3.
Hypothalamic gene expression for the 50% restricted group only
Experiment three: effects on PRH of repleting leptin and TNFα
The indications from experiments one and two are that, under CR, animals remain hungry independent of the length of restriction but highly dependent on its magnitude, which is driven mostly by signals from fat tissue. Of the hormones that we measured, leptin and TNFα might be key, but not necessarily the only, contributors to the hyperphagic response. We finally investigated whether we could diminish the hyperphagia by returning the levels of these hormones to that of a non-restricted animal. During this study we implanted mice with mini-osmotic pumps delivering leptin, TNFα or a placebo [phosphate buffered saline (PBS)] at a dose that aimed to mask the hormonal changes caused by the restriction.
As with the other experiments, there were no significant differences between the control and restricted group in BM, FM, FFM or FI (P>0.05; Fig. 4; supplementary material Table S3) prior to restriction and all significantly decreased during the restriction period (paired t-test: P<0.05). BM decreased by an average of 14.4% (6.4 g), FM by 22.6% (1.8 g) and FFM by 12.7% (4.8 g) in the restricted groups. For control animals, the levels of BM and FFM did not change significantly throughout the study (paired t-test: P>0.05); however, there was a significant gain in FM from 7.5 to 9.3 g (paired t-test: t=2.67, P=0.025).
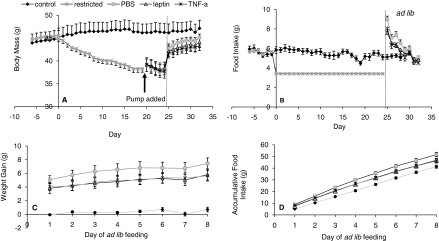
Fig. 4.
BM and FI parameters during the repletion experiment. (A,B) BM and FI are shown. The restricted animals are spilt into three groups after pump implantation. (C,D) Weight gain and accumulative FI after mice were released from restriction onto AL feeding. Data are shown ± s.e.m. See also supplementary material Table S3.
Control animals did not differ in their circulating insulin, leptin or TNFα levels across the three sampling periods conducted throughout the experiment. Mice on restriction had significantly lower circulating leptin, TNFα and insulin compared with controls, before they were implanted with pumps (ANOVA: P<0.05). Mice that were implanted with mini-osmotic pumps delivering leptin had significantly increased leptin levels between the before and after implant measurement (paired t-test: t=3.12, P=0.017) but insulin and TNFα were unchanged. Leptin was increased by the pump manipulation to a level not significantly different from AL feeing controls (ANOVA: F1,16=0.001, P=0.97). For mice implanted with pumps delivering TNFα, there was also a significant increase in circulating TNFα levels between pre- and post-implantation (paired t-test: t=4.80, P=0.002), without changes in leptin and insulin. The resulting levels of circulating TNFα post-implantation were similar to but significantly higher than the AL control animals (12% higher; ANOVA: F1,16=12.39, P=0.003). Mice implanted with pumps delivering PBS did not show any significant differences between the before and after implantation measurements for leptin, TNFα and insulin (P>0.05).
After the animals were released from restriction, they all became hyperphagic (Fig. 4B). The PBS group consumed 9.0 g, the leptin group consumed 7.6 g and the TNFα group consumed 8.0 g on the first day of AL feeding. Intake was significantly higher in the PBS group than the other two (ANOVA: F3,30=27.03, P>0.001). When FI was combined over the entire 8-day AL phase, the leptin (46.8 g) and TNFα (46.2 g) groups had a significantly lower accumulated FI than PBS group (51.7 g) (Fig. 4C). By the end of the study, the daily FI of all three restricted groups was not significantly different to the control animals (ANOVA: F3, 30=0.80, P=0.51).
Owing to the hyperphagia, all three groups significantly gained BM, initially from increased gut fill and subsequently from gains in both FM and FFM. At the end of the AL recovery phase, BM, FM and FFM were not different between any of the four groups (ANOVA: BM, F3,30=0.64, P=0.60; FM, F3,30=0.51, P=0.68; FFM, F3,30=1.33, P=0.28). There had, however, been significantly less BM gain in the leptin and TNFα treatment groups compared with the PBS group (Fig. 4D), which gained approximately 1.7 g more (ANOVA: F2,19=3.81, P=0.041).
DISCUSSION
Our data confirm previous studies that have observed a period of hyperphagia following release from CR. We established here that this hyperphagic response to restriction was independent of the duration of time that the animals were held on restricted intake. The animals never became accustomed to the restricted level of food, despite the fact that restriction for the long-term exposure group was substantial. The period of long-term restriction of 75 days was about 10% of the lifespan of a mouse. Assuming an average lifespan for an MF1 mouse of 740 days (Speakman et al., 2004), this would be equivalent to around 7–8 years of a human’s average lifespan (assuming an average lifespan of 75–80 years) (Speakman and Hambly, 2007). Humans seldom manage to remain on calorie-controlled diet regimes for so long. These mouse data suggest that, whatever the duration of dietary restriction, individuals will not adjust to the new level of intake and will face a constant struggle to remain compliant with dietary restriction interventions.
The extent of PRH also did not increase with increased time spent on restriction, suggesting that this phenomenon was not driven by an acquired knowledge over the restriction period of the growing deficit in intake relative to the AL controls. However, the level of PRH was responsive to the degree of restriction. When mice were given 50% instead of 65% of their initial FI they had a much greater PRH. The level of PRH was therefore heavily dependent on the extent to which body fat and lean tissue had been depleted. When restriction was extended, but without any major change in the depletion of body reserves, there was no further effect on the size of the PRH. When the severity of restriction was increased, but the duration held constant and the reserves were more depleted, there was a greater PRH response. Neuropeptide gene expression, which included increased expression of AgRP and NPY and the decrease in CART and POMC expression in the ARC, indicated elevated hunger in the restricted mice during the restriction phase (Friedman, 2010; Hahn et al., 1998; Schwartz et al., 2000). This pattern remained even after the mice had their food rations for the day, suggesting that they were in a perpetual state of hunger when restricted. Decreased UCP1 expression in BAT suggested that the mice were conserving energy to compensate for the lower energy intake (Hambly and Speakman, 2005).
The sustained hunger neuropeptide profile during restriction, combined with the data on extent and severity of restriction (experiments one and two) on the PRH response suggested that a peripherally generated signal, produced either by the depleted fat or lean tissue, was stimulating appetite and generating the PRH (Dulloo et al., 1997). In experiment two, the level of PRH seemed to be driven by signals from the fat tissue, rather than lean tissue. This is consistent with other studies that showed that aspects of the compensatory response to CR are regulated in relation to depletion of FM (Dulloo and Jacquet, 1998).
Both leptin and TNFα are adipokines that were reduced in restricted mice, and recovered after access to AL food, so we targeted these as potential factors driving the PRH. Moreover, previous work has implicated leptin as a key factor initiating responses to CR (Crujeiras et al., 2010; Friedman, 2010; Rosenbaum et al., 2002; Rosenbaum et al., 2005; Schwartz et al., 2000), whereas both leptin and TNFα are known to regulate appetite via effects on gene expression of the key neuropeptides in the hypothalamus (Endo et al., 2007; Gautron and Elmquist, 2011; Hahn et al., 1998; Kristensen et al., 1998; Schwartz et al., 2000; Woods et al., 2000). Our repletion data (experiment three) clearly show that the PRH response is driven in part by depleted leptin levels in the restricted state. These data are consistent with a large body of evidence relating to the physiological role of leptin in animals and humans. It is well established that low or absent levels of circulating leptin stimulate hyperphagia (Davis et al., 2011; Fam et al., 2007; Friedman and Halaas, 1998; Gautron and Elmquist, 2011; Halaas et al., 1997; Sousa et al., 2009; Schwartz et al., 2000). In humans under CR, weight loss tends to reach a stable plateau after several months of restriction. When leptin was administered to subjects in this weight-stable phase of restriction to replete it to its level in non-weight-reduced individuals, further weight loss occurred (Rosenbaum et al., 2002; Rosenbaum et al., 2005). This was due to a recovery in energy expenditure to the pre-restricted level. When fat stores are depleted, and leptin is reduced, this lowered leptin clearly acts to trigger energy conservation measures and increase appetite. This state can be reversed by returning the levels of circulating leptin to that of a normal unmanipulated individual. In our study, repletion of leptin levels of the restricted animals to the same levels as controls that had not been restricted blunted the PRH by about 16%. This suggested that, although leptin is part of the signalling process driving PRH, it is not responsible for the entire response.
Reduced circulating levels of TNFα acted as an additional signal driving PRH. TNFα is an inflammatory cytokine released by adipose tissue and is becoming recognised as an important component of normal energy homeostasis (Pamir et al., 2009; Endo et al., 2007). It has long been known that elevated TNFα might be an important component of the depressed appetite in disease and infection states (Langhans and Hrupka, 1999; Plata-Salaman et al., 1996; Sonti et al., 1996), such as cancer cachexia (Argiles et al., 2003; Argiles et al., 2005; Bernstein, 1996; Bernstein et al., 1991; Langstein et al., 1991; Smith and Kluger, 1993; Tisdale, 1999), Crohn’s disease (Diamanti et al., 2009), chronic obstructive pulmonary disease (Calikoglu et al., 2004) and infection (Truyens et al., 1995), as well as the anorexia induced by lipopolysaccharide injection (Arsenijevic et al., 2000; Kim et al., 2007; Porter et al., 1998; Tollner et al., 2000). Mice with the TNFα converting enzyme (TACE; also known as ADAM17) inactivated are hypermetabolic and lean (Gelling et al., 2008), whereas those with both TNF receptors knocked out are obese and insulin resistant (Pamir et al., 2009). Injecting TNFα results in a dose-dependent reduction in FI (Fantino and Wieteska, 1993; Kapás and Krueger, 1992; Kapás et al., 1992; Raina and Jeejeebhoy, 1998) mediated via reduced POMC and elevated AgRP in the hypothalamus (Endo et al., 2007), probably involving tyrosine phosphorylation of signal transducer and activator of transcription (STAT) proteins (Rizk et al., 2001; Romanatto et al., 2007). Our data show that reduced levels of TNFα, which accompany fat depletion during CR, stimulate appetite, as reflected in the PRH response, which was blunted when the TNF signal was repleted. At the dosage levels that we used, there was no effect of the infused TNFα on leptin levels, contrasting with other studies that suggest that TNFα regulates leptin production (Grunfeld et al., 1996; Finck and Johnson, 2000; Yamaguchi et al., 1998) either directly or via insulin (Medina et al., 2002). These data suggest a role for TNFα in energy homeostasis under non-pathological conditions, and this role might be independent of peripheral leptin levels but might share central signalling pathways (Langhans and Hrupka, 1999).
The combined data from experimental manipulations of restriction duration and severity, measurements of circulating hormones, tissue gene expression, neuropeptides and, most importantly, the experimental repletion studies presented here, together suggest that, under restriction, it is the depletion of body fat, resulting in lowered leptin and TNFα levels, that in part drives PRH. The discovery of the effects of TNFα in this process are, to our knowledge, novel and support other studies pointing to a role for this adipokine in normal energy homeostasis (Pamir et al., 2009). Compounds targeting TNF receptor populations in the hypothalamus might be valuable adjunct therapies to help people sustain CR for longer, and prevent weight regain in the post-restriction phase.
METHODS
Experiments were carried out on outbred male mice (MF1 strain; Harlan UK): a strain that we have previously extensively characterised in terms of energy balance and response to CR (Hambly et al., 2007a; Hambly et al., 2007b; Hambly and Speakman, 2005; Johnson et al., 2001b). All work was conducted under the UK Home Office Project Licences # 60/3073 and 60/3706. At the onset of each study, mice were 6-months old and were therefore mature adults that were no longer growing. This model is more reflective of adult humans. Mice were housed individually in M3 cages (48×15×13 cm; NKP, Kent, UK) under a 12-hour light:12-hour dark photoperiod at 20±2°C. All mice had AL access to water throughout the study and were provided with wood shavings, a plastic mouse house and shredded paper bedding for enrichment. The diet used in this study was pelleted rat and mouse, breeder and grower diet (Special Diets Services, BP Nutrition, UK), which has a gross energy content of 17.4 MJ/kg (9.2% fat by energy). For all studies, animals were weighed at the same time each day and during periods of restriction this was prior to food provision.
Experiment one: effect of period of restriction on extent of hyperphagia
Over an initial 2-week baseline period, FI and BM were monitored daily in 80 mice fed AL. Previous studies indicate that food spillage for these mice on this diet averages about 2% and can be ignored (Johnson et al., 2001a). Twenty control mice continued feeding AL. The remaining mice (n=60), BM matched to the controls, were placed on CR for 25 days. The restriction level was a reduction to 60% of each animals individually measured FI over baseline. Food was provided at the same time each day in one meal, which was placed directly into the bedding. After 25 days, these mice were assigned to two groups matched for BM (n=30 in each). The groups were either immediately released onto AL feeding for 7 days (short-term restriction) or remained on restriction for an additional 50 days (long-term restriction). The long-term restriction group were then also released from restriction and allowed AL access to food for 7 days. BM and FI were monitored throughout. Because the FI of the control group was not constant, the realised level of restriction relative to control was 62% in the short-term restriction group and 67% in the long-term restriction group. Body composition was determined using magnetic resonance spectroscopy (MRS) (EchoMRI-100; Echo Medical Systems, Houston, TX) on four separate occasions: (1) at the start of the study when all mice were feeding AL, (2) at the end of the short-term restriction, (3) at the end of the long-term restriction and (4) during the final AL phase.
Experiment two: do signals from FM or FFM drive the PRH?
During an initial baseline period of 9 days, 68 mice had their FI and BM measured daily. They also were scanned for FM and FFM on two occasions, using MRS. Mice were then divided into three groups: a control group (n=10) and two restricted groups (n=29 each). Restricted animals were fed either 65% or 50% of their measured baseline daily FI for 25 days. Food was provided in one meal at the same time each day directly into the bedding as previously described. MRS scans were conducted intermittently during this period, prior to feeding.
On day 25 of restriction, the two restricted groups were each further subdivided into three subgroups matched for BM. One subgroup was culled prior to feeding, one subgroup was culled after feeding (n=10 for each restriction level) and one subgroup was provided with AL access to food for 96 hours prior to being culled (n=9). Half of the control mice were culled with the before AL feeding group, and half with the after AL feeding group. The separation of the groups into a ‘before’ and ‘after’ feeding period allowed comparison of the levels of circulating hormones known to alter owing to nutritional status. Blood samples were collected along with samples of WAT (gonadal), BAT, pooled hind leg muscle, liver and brain.
Whole blood was collected in 1 ml Eppendorf tubes containing EDTA as an anticoagulant. The blood was centrifuged and plasma collected and stored at −80°C until analysis. Adipokines were measured using a Luminex system (Luminex Corporation, Austin, TX) employing the mouse adipokine panel to measure circulating levels of leptin, insulin, TNFα, resistin, MCP1 and PAI-1 (Biogenesis, Poole, UK). Circulating levels of IGF1 were measured using enzyme-linked immunosorbent assay (ELISA; IDS, Boldon, UK) and circulating corticosterone was measured using radioimmunoassay (RIA; MP Biomedicals, London, UK).
Tissues were homogenised and total RNA extracted using a guanidium isothiocyanate/phenol method (Chomczynski and Sacchi, 1987). RNA was then separated on a 1.4% denaturing agarose gel prior to capillary blotting onto a positively charged nylon membrane (Amersham Biosciences, Buckinghamshire, UK) overnight. Cross-linked membranes of liver, BAT and muscle samples were hybridised overnight at 42°C using 5′ digoxigenin end-labelled oligonucleotides (Eurogentec) for liver [IGF1 (5′-GATAGGGACGGGGACTTCTGAGTCTTGGGC-3′)], BAT [UCP1 (5′-CGGACTTTGGCGGTGTCCAGCGGGAAGGTGAT-3′)] and muscle [UCP3 (5′-CCCTGACTCCTTCCTCCCTGGCGATGGTTCTG-3′)] mRNA. Gonadal WAT membranes were hybridised for Ob (5′-GGTCTGAGGCAGGGAGCAGCTCTTGGAGAAGGC-3′) and adiponectin (5′-CATACACCTGGAGCCAGACTTGGTC-3′). All membranes were then stripped and hybridised for 18S rRNA (5′-CGCCTGCTGCCTTCCTTGGATGTGGTAGCCG-3′). Signals were detected by chemiluminescence using CDP-Star as the substrate (Tropix) followed by exposure to film. The signals were scanned and quantified by densitometry using ImageJ.
Hypothalamic gene expression was quantified in the 50% restriction group compared with the controls using in situ hybridisation techniques (Simmons et al., 1989). We assessed differences in MC4R in the PVN, and POMC, AgRP, ObRb, CART, NPY and SOCS3 in the ARC. Brain sections were collected onto two slide sets of eight slides per set. The first set of slides spanned the ARC from approximately −2.7 to −1.22 mm relative to Bregma according to the atlas of the mouse brain (Franklin and Paxinos, 1997) and the second set spanned the PVN from −1.22 to −0.46 mm relative to Bregma. After undergoing in situ hybridisation, the slides were exposed to film (Kodak, Biomax MR film) to determine the intensity of the hybridisation signal, which was then quantified using Image Pro Plus (Media Cybernetics) after calibration using a standard curve.
Experiment three: effects on PRH of repleting leptin and TNFα
Thirty-five mice entered this protocol and had BM and FI monitored daily throughout. In addition, a dual-energy x-ray absorptiometry (DXA) scan was conducted to assess FM and FFM on three occasions (baseline, restriction and AL phases). For this scan, mice were anaesthetised using the gaseous anaesthetic isoflurane for the 5-minute duration of the scan and the raw result was corrected using the appropriate equation for our specific machine (Johnston et al., 2005). After a baseline monitoring period of 7 days, the mice were separated into two groups matched for BM. Nine control mice were fed AL for the remainder of the study. The remaining mice were placed on a restricted diet of 60% of their average AL FI for 25 days. At 4 days before the end of the restriction, the mice were implanted with mini-osmotic pumps (Alzet model 2002; pumping rate 0.475 μl/hour) that released recombinant murine TNFα (R&D Systems, UK; n=9), recombinant mouse leptin (R&D Systems, UK; n=10) or a placebo of PBS (Sigma-Aldrich, UK; n=9). On the basis of experiment two, animals received 15.6 μg/day of leptin, or 0.2 μg/day of TNFα. Blood (100μl) was collected prior to and 4 days after pump implantation (tail tip) in EDTA-treated tubes to validate the effectiveness of the pumps. The blood was centrifuged and plasma collected and stored at −80°C until analysis. The mice were then released onto AL feeding for 8 days and culled for further blood collection. Circulating levels of insulin, leptin and TNFα were determined using a Bioplex system (Bio-Rad, CA) with a mouse adipokine panel (Millipore, UK).
Statistics
Data were subjected to ANOVA (Tukey’s) or paired t-tests and are shown ± standard errors where appropriate. P values <0.05 were considered significant. Minitab v15 (Minitab) was used throughout.
TRANSLATIONAL IMPACT.
Clinical issue
The most frequent self- and physician-prescribed treatment for obesity is caloric restriction (CR), or dieting. Although CR yields immediate positive results in terms of reducing both body and fat mass, it is often unsuccessful for long-term obesity treatment because weight is often regained when dieting ceases owing to a phenomenon called post-restriction hyperphagia (PRH). Despite the importance of this phenomenon in the obesity field, our understanding of PRH is surprisingly poor.
Results
In this study, the authors examine PRH in mice after a period of CR and find that reduced fat, rather than altered fat-free mass, is the key factor driving PRH. The main signals from fat mass that drive PRH include reduced levels of tumour necrosis factor-α (TNFα) and leptin during the restriction phase. They also conduct a mini-pump repletion experiment to demonstrate that administering restricted animals with leptin and TNFα so that their levels are equivalent to those in non-restricted animals blunts the PRH response.
Implications and future directions
The discovery that TNFα has an effect on PRH is novel. The data thus indicate that compounds targeting TNF receptor family members in the hypothalamus might be promising candidates for the development of adjunct therapies that will help people sustain CR for longer periods of time and prevent weight regain in the post-restriction phase.
Supplementary Material
Acknowledgments
We thank David Brown, Viv Buchan and Sharon Mitchell for assistance with sample analysis. The Rowett Research Institute and Aberdeen University bio-resources staff provided assistance with animal care and measurements.
Footnotes
FUNDING
This work was supported by SEERAD (Scottish Executive Environment and Rural Affairs Department).
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
C.H. and J.R.S. conceived and designed the experiments. C.H., J.S.D., Z.A.A. and K.M.M. performed the experiments. C.H., J.R.S. and J.G.M. prepared and edited the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.007781/-/DC1
REFERENCES
- Abubakari A. R., Lauder W., Agyemang C., Jones M., Kirk A., Bhopal R. S. (2008). National prevalence of obesity-Prevalence and time trends in obesity among adult West African populations: a meta-analysis. Obes. Rev. 9, 297–311 [DOI] [PubMed] [Google Scholar]
- Allison D. B., Zhu S. K., Plankey M., Faith M. S., Heo M. (2002). Differential associations of body mass index and adiposity with all-cause mortality among men in the first and second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) follow-up studies. Int. J. Obes. Relat. Metab. Disord. 26, 410–416 [DOI] [PubMed] [Google Scholar]
- Andersen L. F., Lillegaard I. T. L., Overby N., Lytle L., Klepp K. I., Johansson L. (2005). Overweight and obesity among Norwegian schoolchildren: changes from 1993 to 2000. Scand. J. Public Health 33, 99–106 [DOI] [PubMed] [Google Scholar]
- Argiles J. M., Moore-Carrasco R., Fuster G., Busquets S., Lopez-Soriano F. J. (2003). Cancer cachexia: the molecular mechanisms. Int. J. Biochem. Cell Biol. 35, 405–409 [DOI] [PubMed] [Google Scholar]
- Argiles J. M., Busquets S., Garcia-Martinez C., Lopez-Soriano F. J. (2005). Mediators involved in the cancer anorexia-cachexia syndrome: past, present, and future. Nutrition 21, 977–985 [DOI] [PubMed] [Google Scholar]
- Aronne L. J., Nelinson D. S., Lillo J. L. (2009). Obesity as a disease state: a new paradigm for diagnosis and treatment. Clin. Cornerstone 9, 9–25 [DOI] [PubMed] [Google Scholar]
- Arsenijevic D., Garcia I., Vesin C., Vesin D., Arsenijevic Y., Seydoux J., Girardier L., Ryffel B., Dulloo A., Richard D. (2000). Differential roles of tumor necrosis factor-alpha and interferon-gamma in mouse hypermetabolic and anorectic responses induced by LPS. Eur. Cytokine Netw. 11, 662–668 [PubMed] [Google Scholar]
- Astrup A., Grunwald G. K., Melanson E. L., Saris W. H. M., Hill J. O. (2000). The role of low-fat diets in body weight control: a meta-analysis of ad libitum dietary intervention studies. Int. J. Obes. 24, 1545–1552 [DOI] [PubMed] [Google Scholar]
- Baldelli R., De Marinis L., D’Amico E., Barnabei A., Pasimeni G., Mecule A., Appetecchia M., Pontecorvi A. (2008). Obesity and cancer. Obe. Metab. 4, 4–11 [Google Scholar]
- Berg C., Rosengren A., Aires N., Lappas G., Toren K., Thelle D., Lissner L. (2005). Trends in overweight and obesity from 1985 to 2002 in Goteborg, West Sweden. Int. J. Obes. 29, 916–924 [DOI] [PubMed] [Google Scholar]
- Bernstein I. L. (1996). Neural mediation of food aversions and anorexia induced by tumor necrosis factor and tumors. Neurosci. Biobehev. Rev. 20, 177–181 [DOI] [PubMed] [Google Scholar]
- Bernstein I. L., Taylor E. M., Bentson K. L. (1991). TNF-induced anorexia and learned food aversions are attenuated by area postrema lesions. Am. J. Physiol. 260, R906–R910 [DOI] [PubMed] [Google Scholar]
- Bradley U., Spence M., Courtney C. H., McKinley M. C., Ennis C. N., McCance D. R., McEneny J., Bell P. M., Young I. S., Hunter S. J. (2009). Low-fat versus low-carbohydrate weight reduction diets effects on weight loss, insulin resistance, and cardiovascular risk: a randomized control trial. Diabetes 58, 2741–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlage P. (2008). Epidemiology and comorbidity of obesity in Germany. Diabetologe 4, 259–265 [Google Scholar]
- Calikoglu M., Sahin G., Unlu A., Ozturk C., Tamer L., Ercan B., Kanik A., Atik U. (2004). Leptin and TNF-alpha levels in patients with chronic obstructive pulmonary disease and their relationship to nutritional parameters. Respiration 71, 45–50 [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate phenol chloroform extraction. Anal. Biochem. 162, 156–159 [DOI] [PubMed] [Google Scholar]
- Crescenzo R., Samec S., Antic V., Rohner-Jeanrenaud F., Seydoux J., Montani J. P., Dulloo A. G. (2003). A role for suppressed thermogenesis favoring catch-up fat in the pathophysiology of catch-up growth. Diabetes 52, 1090–1097 [DOI] [PubMed] [Google Scholar]
- Crescenzo R., Bianco F., Falcone I., Tsalouhidou S., Yepuri G., Mougios V., Dulloo A. G., Liverini G., Iossa S. (2011). Hepatic mitochondrial energetics during catch-up fat with high-fat diets rich in lard or safflower oil. Obesity (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Crujeiras A. B., Goyenechea E., Abete I., Lage M., Carreira M. C., Martínez J. A., Casanueva F. F. (2010). Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J. Clin. Endocrinol. Metab. 95, 5037–5044 [DOI] [PubMed] [Google Scholar]
- Czernichow S., Vergnaud A. C., Teyssier L. M., Peneau S., Bertrais S., Mejean C., Vol S., Tichet J., Hercberg S. (2009). Trends in the prevalence of obesity in employed adults in central-western France: a population-based study, 1995–2005. Prev. Med. 48, 262–266 [DOI] [PubMed] [Google Scholar]
- Das S. K., Saltzman E., Gilhooly C. H., Delany J. P., Golden J. K., Pittas A. G., Dallal G. E., Bhapkar M. V., Fuss P. J., Dutta C., et al. (2009). Low or moderate dietary energy restriction for long-term weight loss: what works best? Obesity 17, 2019–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. F., Choi D. L., Schurdak J. D., Fitzgerald M. F., Clegg D. J., Lipton J. W., Figlewicz J. P., Benoit S. C. (2011). Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol. Psychiatry 69, 668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Corral P., Bryan D. R., Garvey W. T., Gower B. A., Hunter G. R. (2011). Dietary adherence during weight loss predicts weight regain. Obesity 19, 1177–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti A., Basso M., Gambarara M., Papadatou B., Bracci F., Noto C., Castro M. (2009). Positive impact of blocking tumor necrosis factor alpha on the nutritional status in pediatric Crohn’s disease patients. Int. J. Colorectal Dis. 24, 19–25 [DOI] [PubMed] [Google Scholar]
- Doucet E., Cameron J. (2007). Appetite control after weight loss: what is the role of bloodborne peptides? Appl. Physiol. Nutr. Metab. 32, 523–532 [DOI] [PubMed] [Google Scholar]
- Dulloo A. G., Jacquet J. (1998). Adaptive reduction in basal metabolic rate in response to food deprivation in humans: a role for feedback signals from fat stores. Am. J. Clin. Nutr. 68, 599–606 [DOI] [PubMed] [Google Scholar]
- Dulloo A. G., Jacquet J., Girardier L. (1996). Autoregulation of body composition during weight recovery in human: The Minnesota Experiment revisited. Int. J. Obes. Relat. Metab. Disord. 20, 393–405 [PubMed] [Google Scholar]
- Dulloo A. G., Jacquet J., Girardier L. (1997). Poststarvation hyperphagia and body fat overshooting in humans: a role for feedback signals from lean and fat tissues. Am. J. Clin. Nutr. 65, 717–723 [DOI] [PubMed] [Google Scholar]
- Dulloo A. G., Jacquet J., Seydoux J., Montani J. P. (2006). The thrifty ‘catch-up fat’ phenotype: its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int. J. Obes. 30, S23–S35 [DOI] [PubMed] [Google Scholar]
- Endo M., Masaki T., Seike M., Yoshimatsu H. (2007). Involvement of stomach ghrelin and hypothalamic neuropeptides in tumor necrosis factor-alpha-induced hypophagia in mice. Regul. Pept. 140, 94–100 [DOI] [PubMed] [Google Scholar]
- Fam B. C., Morris M. J., Hansen M. J., Kebede M., Andrikopoulos S., Proietto J., Thorburn A. W. (2007). Modulation of central leptin sensitivity and energy balance in a rat model of diet-induced obesity. Diabetes Obes. Metab. 9, 840–852 [DOI] [PubMed] [Google Scholar]
- Fantino M., Wieteska L. (1993). Evidence for a direct central anorectic effect of tumor-necrosis-factor-alpha in the rat. Physiol. Behav. 53, 477–483 [DOI] [PubMed] [Google Scholar]
- Field A. E., Camargo C. A., Taylor C. B., Berkey C. S., Roberts S. B., Colditz G. A. (2001). Peer, parent, and media influences on the development of weight concerns and frequent dieting among preadolescent and adolescent girls and boys. Pediatrics 107, 54–60 [DOI] [PubMed] [Google Scholar]
- Finck B. N., Johnson R. W. (2000). Tumor necrosis factor-alpha regulates secretion of the adipocyte-derived cytokine, leptin. Microsc. Res. Tech. 50, 209–215 [DOI] [PubMed] [Google Scholar]
- Finkelstein E. A., Trogdon J. G., Cohen J. W., Dietz W. (2009). Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Affairs 28, w822–w831 [DOI] [PubMed] [Google Scholar]
- Flegal K. M., Graubard B. I. (2009). Estimates of excess deaths associated with body mass index and other anthropometric variables. Am. J. Clin. Nutr. 89, 1213–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal K. M., Carroll M. D., Ogden C. L., Johnson C. L. (2002). Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288, 1723–1727 [DOI] [PubMed] [Google Scholar]
- Flegal K. M., Ogden C. L., Carroll M. D. (2004). Prevalence and trends in overweight in Mexican-American adults and children. Nutr. Rev. 62, S144–S148 [DOI] [PubMed] [Google Scholar]
- Flegal K. M., Graubard B. I., Williamson D. F., Gail M. H. (2007a). Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 298, 2028–2037 [DOI] [PubMed] [Google Scholar]
- Flegal K. M., Graubard B. I., Williamson D. F., Gail M. H. (2007b). Weight-associated deaths in the United States. J. Womans Health 16, 1368–1370 [DOI] [PubMed] [Google Scholar]
- Flegal K. M., Carroll M. D., Ogden C. L., Curtin L. R. (2010). Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303, 235–241 [DOI] [PubMed] [Google Scholar]
- Franklin K. B. J., Paxinos G. (1997). The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press [Google Scholar]
- Friedman J. M. (2010). A tale of two hormones. Nat. Med. 16, 1100–1006 [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Halaas J. L. (1998). Leptin and the regulation of body weight in mammals. Nature 395, 763–770 [DOI] [PubMed] [Google Scholar]
- Frisch R. E. (1993). Critical fat. Science 261, 1103–1104 [DOI] [PubMed] [Google Scholar]
- Frisch R. E. (1994). The right weight-body-fat, menarche and fertility. Proc. Nutr. Soc. 53, 113–129 [DOI] [PubMed] [Google Scholar]
- Frisch R. E. (1996). The right weight: body fat, menarche, and fertility. Nutrition 12, 452–453 [DOI] [PubMed] [Google Scholar]
- Gautron L., Elmquist J. K. (2011). Sixteen years and counting: an update on leptin in energy balance. J. Clin. Invest. 121, 2087–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelling R. W., Yan W. B., Al-Noori S., Pardini A., Morton G. J., Ogimoto K., Schwartz M. W., Dempsey P. J. (2008). Deficiency of TNF alpha converting enzyme (TACE/ADAM17) causes a lean, hypermetabolic phenotype in mice. Endocrinology 149, 6053–6064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C., Zhao C., Fuller J., Pollock A., Moser A., Friedman J., Feingold K. R. (1996). Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters – A role for leptin in the anorexia of infection. J. Clin. Invest. 97, 2152–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T. M., Breininger J. F., Baskin D. G., Schwartz M. W. (1998). Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1, 271–272 [DOI] [PubMed] [Google Scholar]
- Halaas J. L., Boozer C., BlairWest J., Fidahusein N., Denton D. A., Friedman J. M. (1997). Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. USA 94, 8878–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambly C., Speakman J. R. (2005). Contribution of different mechanisms to compensation for energy restriction in the mouse. Obes. Res. 13, 1548–1557 [DOI] [PubMed] [Google Scholar]
- Hambly C., Mercer J. G., Speakman J. R. (2007a). Hunger does not diminish over time in mice under protracted caloric restriction. Rejuvenation Res. 10, 533–541 [DOI] [PubMed] [Google Scholar]
- Hambly C., Simpson C. A., McIntosh S., Duncan J. S., Dalgleish G. D., Speakman J. R. (2007b). Calorie-restricted mice that gorge show less ability to compensate for reduced energy intake. Physiol. Behav. 92, 985–992 [DOI] [PubMed] [Google Scholar]
- Jackman M. R., Steig A., Higgins J. A., Johnson G. C., Fleming-Elder B. K., Bessesen D. H., MacLean P. S. (2008). Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1117–R1129 [DOI] [PubMed] [Google Scholar]
- Johnson M. S., Thomson S. C., Speakman J. R. (2001a). Limits to sustained energy intake I. Lactation in the laboratory mouse Mus musculus. J. Exp. Biol. 204, 1925–1935 [DOI] [PubMed] [Google Scholar]
- Johnson M. S., Thomson S. C., Speakman J. R. (2001b). Limits to sustained energy intake II. Inter-relationships between resting metabolic rate, life-history traits and morphology in Mus musculus. J. Exp. Biol. 204, 1937–1946 [DOI] [PubMed] [Google Scholar]
- Johnston S. L., Peacock W. L., Bell L. M., Lonchampt M., Speakman J. R. (2005). PIXImus DXA with different software needs individual calibration to accurately predict fat mass. Obes. Res. 13, 1558–1565 [DOI] [PubMed] [Google Scholar]
- Kain J., Uauy R., Vio F., Albala C. (2002). Trends in overweight and obesity prevalence in Chilean children: comparison of three definitions. Eur. J. Clin. Nutr. 56, 200–204 [DOI] [PubMed] [Google Scholar]
- Kapás L., Krueger J. M. (1992). Tumor necrosis Factor-Beta induces sleep, fever, and anorexia. Am. J. Physiol. 263, R703–R707 [DOI] [PubMed] [Google Scholar]
- Kapás L., Hong L. L., Cady A. B., Opp M. R., Postlethwaite A. E., Seyer J. M., Krueger J. M. (1992). Somnogenic, pyrogenic, and anorectic activities of tumor-necrosis-factor-alpha and Tnf-alpha fragments. Am. J. Physiol. 263, R708–R715 [DOI] [PubMed] [Google Scholar]
- Kim Y. W., Kim K. H., Ahn D. K., Kim H. S., Kim J. Y., Lee D. C., Park S. Y. (2007). Time-course changes of hormones and cytokines by lipopolysaccharide and its relation with anorexia. J. Physiol. Sci. 57, 159–165 [DOI] [PubMed] [Google Scholar]
- Kristensen P., Judge M. E., Thim L., Ribel U., Christjansen K. N., Wulff B. S., Clausen J. T., Jensen P. B., Madsen O. D., Vrang N., et al. (1998). Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393, 72–76 [DOI] [PubMed] [Google Scholar]
- Labayen I., Ortega F. B., Ruiz J. R., Lasa A., Simon E., Margareto J. (2011). Role of baseline leptin and ghrelin levels on body weight and fat mass changes after an energy-restricted diet intervention in obese women: effects on energy metabolism. J. Clin. Endocrinol. Metab. 96, E996–E1000 [DOI] [PubMed] [Google Scholar]
- Langhans W., Hrupka B. (1999). Interleukins and tumor necrosis factor as inhibitors of food intake. Neuropeptides 33, 415–424 [DOI] [PubMed] [Google Scholar]
- Langstein H. N., Doherty G. M., Fraker D. L., Buresh C. M., Norton J. A. (1991). The roles of gamma-interferon and tumor-necrosis-factor-alpha in an experimental rat model of cancer cachexia. Cancer Res. 51, 2302–2306 [PubMed] [Google Scholar]
- Leibel R. L., Rosenbaum M., Hirsch J. (1995). Changes in energy-expenditure resulting from altered body-weight. N. Engl. J. Med. 332, 621–628 [DOI] [PubMed] [Google Scholar]
- Lowry R., Galuska D. A., Fulton J. E., Burgeson C. R., Kann L. (2005). Weight management goals and use of exercise for weight control among U.S. high school students, 1991–2001. J. Adolesc. Health 36, 320–326 [DOI] [PubMed] [Google Scholar]
- MacLean P. S. (2005). A peripheral perspective of weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R1447–R1449 [DOI] [PubMed] [Google Scholar]
- MacLean P. S., Higgins J. A., Johnson G. C., Fleming-Elder B. K., Donahoo W. T., Melanson E. L., Hill J. O. (2004). Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R1306–R1315 [DOI] [PubMed] [Google Scholar]
- MacLean P. S., Higgins J. A., Jackman M. R., Johnson G. C., Fleming-Elder B. K., Wyatt H. R., Melanson E. L., Hill J. O. (2006). Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1577–R1588 [DOI] [PubMed] [Google Scholar]
- MacLean P. S., Bergouignan A., Cornier M. A., Jackman M. R. (2011). Biology’s response to dieting: the impetus for weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann T., Tomiyama A. J., Lew A. M., Westling E., Chatman J., Samuels B. (2007). The search for effective obesity treatments: should Medicare fund diets? Am. Psychol. 62, 220–233 [DOI] [PubMed] [Google Scholar]
- Mark A. L. (2008). Dietary therapy for obesity: and emperor with no clothes. Hypertension 51, 1426–143418474832 [Google Scholar]
- Medina E. A., Erickson K. L., Stanhope K. L., Havel P. J. (2002). Evidence that tumor necrosis factor-alpha-induced hyperinsulinemia prevents decreases of circulating leptin during fasting in rats. Metabolism 51, 1104–1110 [DOI] [PubMed] [Google Scholar]
- Nock N. L., Thompson C. L., Tucker T. C., Berger N. A., Li L. (2008). Associations between obesity and changes in adult BMI over time and colon cancer risk. Obesity 16, 1099–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Costa F., Rocha G. Z., Dias M. M., Carvalheira J. B. C. (2009). Epidemiological and molecular mechanisms aspects linking obesity and cancer. Arq. Bras. Endocrinol. Metab. 53, 213–226 [DOI] [PubMed] [Google Scholar]
- Pamir N., McMillen T. S., Kaiyala K. J., Schwartz M. W., LeBoeuf R. C. (2009). Receptors for tumor necrosis factor-alpha play a protective role against obesity and alter adipose tissue macrophage status. Endocrinology 150, 4124–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata-Salamán C. R., Sonti G., Borkoski J. P., Wilson C. D., FfrenchMullen J. M. H. (1996). Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol. Behav. 60, 867–875 [PubMed] [Google Scholar]
- Porter M. H., Arnold M., Langhans W. (1998). TNF-alpha tolerance blocks LPS-induced hypophagia but LPS tolerance fails to prevent TNF-alpha-induced hypophagia. Am. J. Physiol. 274, R741–R745 [DOI] [PubMed] [Google Scholar]
- Raina N., Jeejeebhoy K. N. (1998). Changes in body composition and dietary intake induced by tumor necrosis factor alpha and corticosterone – individually and in combination. Am. J. Clin. Nutr. 68, 1284–1290 [DOI] [PubMed] [Google Scholar]
- Raj M., Sundaram K. R., Paul M., Deepa A. S., Kumar R. K. (2007). Obesity in Indian children: time trends and relationship with hypertension. Natl. Med. J. India 20, 288–293 [PubMed] [Google Scholar]
- Ravussin Y., Gutman R., Diano S., Shanabrough M., Borok E., Sarman B., Lehmann A., LeDuc C. A., Rosenbaum M., Horvath T. L., et al. (2011). Effects of chronic weight pertubation on energy homeostasis and brain structure in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1352–R1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk N. M., Stammsen D., Preibisch G., Eckel J. (2001). Leptin and tumor necrosis factor-alpha induce the tyrosine phosphorylation of signal transducer and activator of transcription proteins in the hypothalamus of normal rats in vivo. Endocrinology 142, 3027–3032 [DOI] [PubMed] [Google Scholar]
- Romanatto T., Cesquini M., Amaral M. E., Roman E. A., Moraes J. C., Torsoni M. A., Cruz A. P., Velloso L. A. (2007). TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient – Effects on leptin and insulin signaling pathways. Peptides 28, 1050–1058 [DOI] [PubMed] [Google Scholar]
- Rosenbaum M., Murphy E. M., Heymsfield S. B., Matthews D. E., Leibel R. L. (2002). Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J. Clin. Endocrinol. Metab. 87, 2391–2394 [DOI] [PubMed] [Google Scholar]
- Rosenbaum M., Vandenborne K., Goldsmith R., Simoneau J. A., Heymsfield S., Joanisse D. R., Hirsch J., Murphy E., Matthews D., Segal K. R., et al. (2003). Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R183–R192 [DOI] [PubMed] [Google Scholar]
- Rosenbaum M., Goldsmith R., Bloomfield D., Magnano A., Weimer L., Heymsfield S., Gallagher D., Mayer L., Murphy E., Leibel R. L. (2005). Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Invest. 115, 3579–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M., Hirsch J., Gallagher D. A., Leibel R. L. (2008). Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am. J. Clin. Nutr. 88, 906–912 [DOI] [PubMed] [Google Scholar]
- Sanyal A. J. (2011). NASH: a global health problem. Hepatol. Res. 41, 670–674 [DOI] [PubMed] [Google Scholar]
- Schneider J. E. (2004). Energy balance and reproduction. Physiol. Behav. 81, 289–317 [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Zhou D., Blum R. M. (2000). Leptin and metabolic control of reproduction. Horm. Behav. 37, 306–326 [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Woods S. C., Porte D., Seeley R. J., Baskin D. G. (2000). Central nervous system control of food intake. Nature 404, 661–671 [DOI] [PubMed] [Google Scholar]
- Simmons D. M., Arriza J. L., Swanson L. W. (1989). A Complete protocol for insitu hybridization of messenger-RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J. Histotechnol. 12, 169–181 [Google Scholar]
- Smith B. K., Kluger M. J. (1993). Anti-Tnf-alpha antibodies normalized body-temperature and enhanced food-intake in tumor-bearing rats. Am. J. Physiol. 265, R615–R619 [DOI] [PubMed] [Google Scholar]
- Sonti G., Ilyin S. E., PlataSalaman C. R. (1996). Anorexia induced by cytokine interactions at pathophysiological concentrations. Am. J. Physiol. 270, R1394–R1402 [DOI] [PubMed] [Google Scholar]
- Sousa M., Bras-Silva C., Leite-Moreira A. (2009). The role of leptin in the regulation of energy balance. Acta Med. Port. 22, 291–298 [PubMed] [Google Scholar]
- Speakman J. R., Hambly C. (2007). Starving for life: what animal studies can and cannot tell us about the use of caloric restriction to prolong human lifespan J. Nutr. 137, 1078–1086 [DOI] [PubMed] [Google Scholar]
- Speakman J. R., Talbot D. A., Selman C., Snart S., McLaren J. S., Redman P., Krol E., Jackson D. M., Johnson M. S., Brand M. D. (2004). Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 3, 87–95 [DOI] [PubMed] [Google Scholar]
- Tisdale M. J. (1999). Wasting in cancer. J. Nutr. 129, 243S–246S [DOI] [PubMed] [Google Scholar]
- Tollner B., Roth J., Storr B., Martin D., Voigt K., Zeisberger E. (2000). The role of tumor necrosis factor (TNF) in the febrile and metabolic responses of rats to intraperitoneal injection of a high dose of lipopolysaccharide. Pflugers Arch. 440, 925–932 [DOI] [PubMed] [Google Scholar]
- Truyens C., Torrico F., AngeloBarrios A., Lucas R., Heremans H., DeBaetselier P., Carlier Y. (1995). The cachexia associated with Trypanosoma cruzi acute infection in mice is attenuated by anti-TNF-alpha, but not by anti-IL-6 or anti-IFN-gamma antibodies. Parasite Immunol. 17, 561–568 [DOI] [PubMed] [Google Scholar]
- Tsai A. G., Williamson D. F., Glick H. A. (2011). Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obes. Rev. 12, 50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade G. N., Schneider J. E. (1992). Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev. 16, 235–272 [DOI] [PubMed] [Google Scholar]
- Wolf A. M., Colditz G. A. (1998). Current estimates of the economic cost of obesity in the United States. Obes. Res. 6, 97–106 [DOI] [PubMed] [Google Scholar]
- Woods S. C., Schwartz M. W., Baskin D. G., Seeley R. J. (2000). Food intake and the regulation of body weight. Annu. Rev. Psychol. 51, 255–277 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Murakami T., Tomimatsu T., Nishio Y., Mitsuda N., Kanzaki T., Kurachi H., Shima K., Aono T., Murata Y. (1998). Autocrine inhibition of leptin production by tumor necrosis factor-alpha (TNF-alpha) through TNF-alpha type-I receptor in vitro. Biochem. Biophys. Res. Commun. 244, 30–34 [DOI] [PubMed] [Google Scholar]
- Yepuri G., Marcelino H., Shahkhalili Y., Aprikian O., Macé K., Seydoux J. L., Miles J., Montani J. P., Dulloo A. G. (2011). Dietary modulation of body composition and insulin sensitivity during catch-up growth in rats: effects of oils rich in n-6 or n-3 PUFA. Br. J. Nutr. 31, 1–14 [DOI] [PubMed] [Google Scholar]
- Zhu S. K., Wang Z. M., Heshka S., Heo M. S., Faith M. S., Heymsfield S. B. (2002). Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am. J. Clin. Nutr. 76, 743–749 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.