SUMMARY
γ-secretase inhibitors (GSIs) have been recently proposed as chemopreventive agents in gastrointestinal neoplasia, because they lead, through inhibition of the Notch signaling pathway, to goblet cell conversion in some intestinal adenomas of the ApcMin mice, and halt epithelial cell proliferation. In this study, we examine in depth, in normal mice, the effects of a GSI, dibenzazepine (DBZ), intraperitoneally administered for 8 days at a non toxic dose, on the gene expression pattern of secretory mucin (MUC), goblet cell conversion, organization of the crypt structural-proliferative units, stem cell niche and apoptotic compartments, along the entire length of the small intestine and colon. We demonstrate that DBZ elicits a homogeneous goblet cell conversion all along the mouse intestinal tract, associated with an overexpression of the gene Muc2 without ectopic expression of the gastric genes Muc5ac and Muc6, and with the emergence of lysozyme-positive ‘intermediate cells’ in the colon. Furthermore, DBZ treatment induces a heterogeneous reorganization of the crypt structural-proliferative units along the intestinal tract and of the stem cell niche in the colon, without disturbing the apoptotic compartment. These findings point to uncoupled effects of a GSI on goblet cell conversion and reorganization of the intestinal crypt structural-proliferative units and stem cell niche, and suggest caution in the use of GSIs as chemopreventive agents for intestinal neoplasia.
INTRODUCTION
The self renewing intestinal epithelium is ordered into structural proliferating and differentiated units that are tightly regulated by signaling pathways, including the γ-secretase-Notch pathway (Van der Flier and Clevers, 2009). γ-secretase is a multi-protein complex that proteolyzes the transmembrane region of the Notch-receptor intracellular domain (NICD), and of other proteins such as the amyloid precursor protein (APP) and CD44 in various cell types (for a review, see Artavanis-Tsakonas et al., 1999; Iwatsubo, 2004). Recently, γ-secretase inhibitors (GSIs) have been developed, which are of considerable interest because of their potential use in several disease states including Alzheimer’s disease and cancer. Much of their pharmacological interest stems from the fact that they target the Notch signaling pathway. In particular, because aberrant Notch signaling has been implicated in tumorigenesis, GSIs have been proposed as chemopreventive and/or chemotherapeutic agents in colon cancer (Meng et al., 2009), Barrett’s esophagus (Menke et al., 2010) and several non gastrointestinal cancers (Real et al., 2009; Wei et al., 2010; Al-Husaini et al., 2011). This therapeutic potential of GSIs is based on Notch signaling being essential to maintain the undifferentiated, proliferative state of intestinal crypt progenitors (Fre et al., 2005). By inhibiting Notch signaling, GSIs lead to an increase in the BHLH transcription factor Math1 (also known as ATOH1; an important factor for epithelial cell fate determination, which is repressed by Notch activation) and to a massive conversion of crypt epithelial cells into goblet cells in the murine intestine (Milano et al., 2004; van Es et al., 2005; Okamoto et al., 2009). GSIs also induce goblet cell differentiation in some intestinal adenomas of mice with a mutation in the Apc tumor suppressor gene (ApcMin mice) (van Es et al., 2005). This effect on epithelial differentiation has been shown to be associated with a cessation in cell division, i.e. with the loss of Ki67-positive cells at the crypt base in the small intestine of normal mice, and in the adenomas of the ApcMin mice (van Es et al., 2005). In other words, GSIs induce a Math1-positive, goblet cell (Alcian Blue positive, PAS positive), Ki67-negative phenotype in the crypt epithelial cells of the small intestine.
In this context, the aim of the present study was to examine whether the phenotypic conversion of epithelial cells elicited by GSIs is a homogeneous or heterogeneous feature along the length of the mouse intestinal tract. To this end, we examined the effects on the whole intestinal tract (from duodenum to left colon) of a well-known γ-secretase inhibitor, dibenzazepine (DBZ), administrated to mice daily for 8 days at an efficient and nontoxic dose. Using a multiparametric approach, we studied the effects of DBZ on the expression of secretory mucin (MUC) genes, on the conversion of epithelial cells into goblet cells and on the disorganization of the crypt proliferative compartment. This was assessed both in situ and on the different fractions of the colonic crypts after sequential isolation of colonic epithelial cells, using Ki67 immunostaining and markers of the stem cell niche. We demonstrate that, although γ-secretase inhibition elicits a homogeneous goblet cell conversion and overexpression of the Muc2 gene only, all along the mouse intestinal tract, it has heterogeneous effects on the structural-proliferative units of intestinal crypts – the majority of crypts displaying an upward shift of the proliferative compartment – paralleled by an alteration of stem cell activity in the colon, and does not disturb the apoptotic compartment. These findings point to a differential sensitivity of the intestinal secretory lineage and the crypt renewal (proliferative) status to γ-secretase inhibition.
RESULTS
Expression of Muc and Math1 mRNA along the mouse gastrointestinal tract
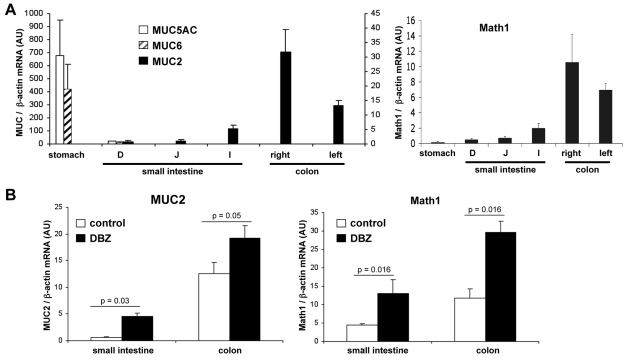
We first determined the expression profile of secretory MUC genes along the gastrointestinal tract (GIT) of wt C57BL6 mice. To this end, quantitative RT-PCR (Q-PCR) was performed after RNA extraction from the stomach and the various regions of small intestine (duodenum, jejunum, ileum) and colon (right and left colon). As shown in Fig. 1A, Muc5ac and Muc6 mRNAs were restricted to the stomach, and not expressed in the small intestine and colon. Conversely, Muc2 mRNAs were not detected in the stomach, but expressed along the small intestine and colon, with a maximal expression in the right colon (Fig. 1A, left panel). Math1 mRNA was hardly detectable in the stomach, and paralleled that of Muc2 in the small intestine and colon (Fig. 1A, right panel).
Fig. 1.
Expression of various Muc and Math1 mRNAs along the entire mouse gastrointestinal tract of normal mice and mice treated with the GSI DBZ. (A,B) Muc and Math1 mRNAs levels were quantified by Q-PCR and expressed relative to the levels of β-actin mRNA. Values are means ± s.e.m. of normal C57BL6 mice (A; n=4), and of mice treated or not with DBZ (5 μmol/kg, daily intraperitoneal injection for 8 days; B; n=4 per condition).
Regulation of Muc2 and Math1 mRNA levels by the γ-secretase inhibitor DBZ
To evaluate the in vivo effects of γ-secretase inhibition on Math1 and Muc gene expression along the intestine and colon, DBZ was administered to C57BL6 mice by daily intraperitoneal injections of 5 μmol/kg for 8 days. At this dose, DBZ was nontoxic, as the mice did not display any weight loss, neurological signs, or diarrhea. As shown in Fig. 1B, DBZ significantly increased Muc2 mRNA levels compared with the level in control mice, in the small intestine (threefold increase over the controls) and colon (1.5-fold increase). In parallel, Math1 mRNA levels were greatly increased in both small intestine and colon compared with controls (threefold increase; Fig. 1B). Muc5ac and Muc6 mRNAs remained undetectable in the small intestine and colon after DBZ treatment. Results were similar in the proximal small intestine and colon (duodenum and right colon; Fig. 1B) and in the distal small intestine and colon (ileum and left colon).
Effect of DBZ treatment on the secretory phenotype of epithelial cells in the small intestine and colon
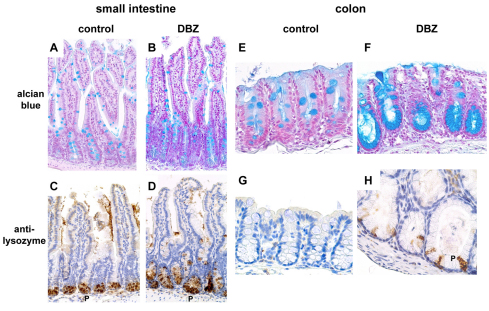
We assessed morphologically the effects of DBZ treatment on two major secretory phenotypes of intestinal epithelial cells: mucus production, visualized by Alcian Blue staining, and lysozyme production (by immunostaining), a feature of Paneth cells, normally found only in the base of the crypts of Lieberkühn in the small intestine. Alcian-Blue-positive cells substantially increased in the small intestine upon DBZ treatment (Fig. 2B) compared with those in control mice (Fig. 2A), in the elongated crypts and to a lesser extent in the villi, and greatly increased in the colon, mainly at the base of the enlarged crypts (Fig. 2E,F). Remarkably, in the colon, all crypts exhibited a massive conversion of epithelial cells into Alcian-Blue-positive goblet cells (Fig. 2F). The number of Paneth cells, visualized by lysozyme immunostaining (Fig. 2C,D), increased in the small intestine of DBZ-treated mice [9±0.5 (mean ± s.e.m.) lysozyme-positive cells per crypt in DBZ-treated mice versus 5.3±0.07 positive cells per crypt in control mice; P=0.02; n=4 mice per condition]. Surprisingly, a few lysozyme-positive cells appeared focally in the colon in DBZ-treated mice, interspersed with mucus-secreting cells in the dilated crypts (Fig. 2H), whereas they were never found in control mice (Fig. 2G). The number of lysozyme-positive cells was 27±15 per 100 colonic crypts in DBZ-treated mice, and 0 in control mice (n=4 mice per condition).
Fig. 2.
Effect of DBZ on the secretory functions of mouse small intestine and colon. Small intestine and colon sections of control and DBZ-treated mice were stained with Alcian Blue (A,B,E,F) or immunostained with anti-lysozyme antibody (brown; C,D,G,H). DBZ treatment elicited an increase in Alcian-Blue-positive goblet cells of the small intestine (B) and colonic crypts (F) compared with control mice (A,E). DBZ also led to an increase in lysozyme-positive Paneth cells (P) in the small intestine (D) compared to controls (C), and to an ectopic expression of Paneth cells in the colon (H). Images are representative of eight mice per group. Original magnification 200×.
Effect of DBZ treatment on the organization of the crypt structural-proliferative units along the intestinal tract
The organization of the crypt renewal (proliferative) compartment was examined in both small intestine and colon in two ways: (1) the organization of the transit-amplifying compartment was assessed by Ki67 immunostaining; (2) the organization of the stem cell niche was examined by assessing, (i) the topography of CD24-positive cells, which provide essential support to the leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5) stem cells (Sato et al., 2011), and (ii) the Lgr5 mRNA expression levels in the isolated fractions of colonic crypts.
Ki67 immunolabeling
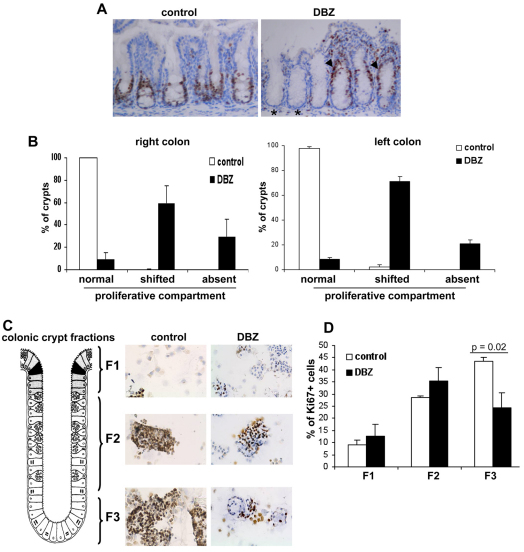
In both the small intestine (not shown), and in the right and left colon (Fig. 3A,B), DBZ treatment led to a redistribution of the proliferative compartment, as determined by Ki67 staining. In control mice, Ki67-positive cells were restricted to the crypt base (Fig. 3A). In the right colon of DBZ-treated mice, only 10% of crypts had Ki67-positive cells in the normal location (predominant at the crypt base), 30% of crypts were devoid of Ki67-positive cells and in 60% of the crypts the Ki67-positive cells had shifted to the upper two-thirds of the crypts (Fig. 3A,B). The results were similar in the left colon (Fig. 3B, right). To obtain more insight into the effects of DBZ on proliferation in the different fractions of the colonic crypt, we performed a fractionation of colonic epithelial cells from the surface (fraction 1, named F1) to the base of crypts (fraction 3; F3). In control mice, Ki67 immunostaining of cytospin preparations of the three fractions showed, as expected, the highest number of positive cells in F3 (Fig. 3C,D). In DBZ-treated mice, there was a 50% significant decrease in Ki67-positive cells in F3 compared with control mice (Fig. 3D). Moreover, DBZ treatment led to an overall decrease in Ki67-positive cells of ∼20%. These findings paralleled the immunostaining on whole tissues.
Fig. 3.
Heterogeneous effect of DBZ on the structural-proliferative units of mouse colonic crypts. (A) Sections of colon from control and DBZ-treated mice were immunostained with anti-Ki67 antibody (A; Ki67-positive cells are brown) and counterstained with Hematoxylin (blue). Ki67-positive cells are located at the base of crypts in control mice. DBZ elicited a loss of Ki67+ cells in some colonic crypts (*) and an upward shift of Ki67-positive cells in the majority of colonic crypts (arrowheads). (B) Percentage of crypts with normal, upward shifted or absent proliferative cells, in both the right and left colon, as determined by counting Ki67-positive crypts as described in the Methods. (C,D) Colonic crypt fractions (F1–3) were obtained after EDTA treatment. Cytospin preparations of the three fractions were immunostained with Ki67 (C), and the percentage of Ki67-positive cells in each fraction was calculated (D). Values are means ± s.e.m. of four mice per group (B,D). Original magnification 200×.
CD24 and Lgr5 expression
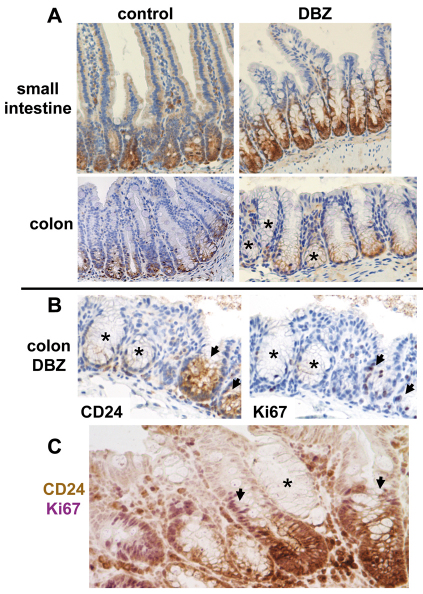
In the small intestine of control mice, CD24 expression was restricted to cells at the crypt base, including Paneth cells. The same pattern of expression was observed in the small intestine of DBZ-treated mice, and was present in all crypts (Fig. 4A, upper panel). In the colon of control mice, CD24 was restricted to the base of all crypts. By contrast, in DBZ-treated mice, a heterogeneous labeling was observed, with some crypts devoid of CD24 labeling (Fig. 4A, lower panel). Interestingly, in crypts maintaining CD24 expression, positive cells were restricted to the base (Fig. 4A, lower panel). CD24 and Ki67 stainings on serial sections (Fig. 4B), as well as double immunostaining for CD24 and Ki67 (Fig. 4C) showed that the majority of CD24-negative crypts were also Ki67 negative. Indeed, counting at least 100 crypts on double stained sections showed that 17±2% crypts were CD24 negative and 80±5% of them were Ki67 negative (mean ± s.e.m. of four DBZ-treated mice).
Fig. 4.
Effect of DBZ treatment on the stem cell niche. (A) Small intestine or colon sections of control and DBZ-treated mice were immunostained with CD24 and counterstained with Hematoxylin. In the small intestine, CD24-positive cells (brown), including Paneth cells, were found at the base of all crypts both in control and DBZ-treated mice (upper panel). In the colon of control mice, CD24-positive cells were also restricted to the base of all crypts, whereas in DBZ-treated mice they were absent from some crypts (*; lower panel). (B) staining of CD24 and Ki67 on serial sections of the colon from DBZ-treated mice showed that the majority of crypts devoid of CD24 were Ki67 negative (*). Ki67-positive cells were restricted to CD24-positive crypts (arrows). (C) Sequential double staining for CD24 (brown) and Ki67 (purple) on a section from the colon of a DBZ-treated mouse. A CD24-negative, Ki67-negative crypt is shown (*), as well as CD24-positive, Ki67-positive crypts with an upward shift of Ki67-positive cells (arrows: purple nuclei above the CD24-positive staining at the crypt base). Representative images of four mice. Original magnification 200×.
To examine whether this alteration of CD24 expression in the colon of DBZ-treated mice was paralleled by an alteration of stem cell activity, we studied Lgr5 mRNA levels in the isolated fractions of colonic crypts. As shown in Fig. 5, Lgr5 mRNAs were mainly found in the F3 fraction of colonic crypts in control mice. DBZ treatment led to a significant downregulation of Lgr5 mRNA levels in the F3 fraction (Fig. 5).
Fig. 5.
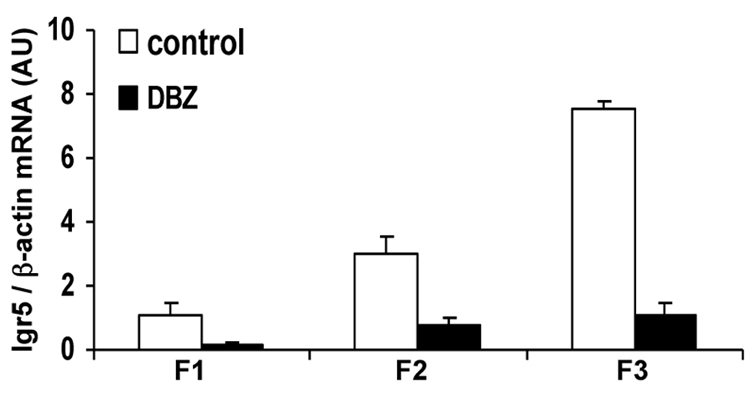
Effect of DBZ treatment on Lgr5 expression. Lgr5 mRNA levels were quantified by Q-PCR in fractions of isolated colonic crypts from control and DBZ-treated mice, and expressed relative to β-actin levels (means ± s.e.m. of four mice per group).
Effect of DBZ on epithelial apoptosis of mouse small intestine and colon
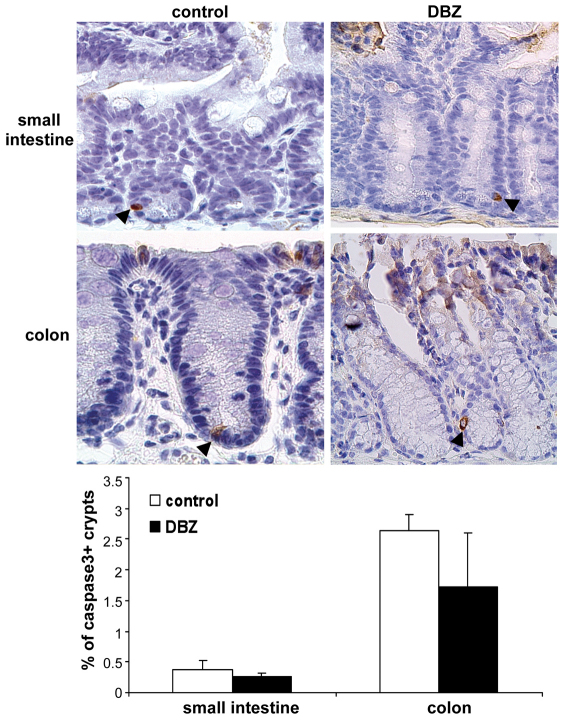
In order to assess apoptosis, immunostaining of active caspase-3 was performed on mouse small intestine and colon sections. In control mice, caspase-3 immunostaining was observed in the surface exfoliating epithelial cells, serving as an internal positive control. We particularly focused on caspase-3-positive cells at the crypt base. In control mice, crypt bases containing at least one caspase-3-positive cell were rare in small intestine and colon (Fig. 6). In DBZ-treated mice, no significant difference in the percentage of caspase-3-positive crypts was noted, either in the small intestine or in the colon (Fig. 6).
Fig. 6.
DBZ does not modify the apoptotic compartments in the mouse small intestine and colon. Small intestine and colon sections of control and DBZ-treated mice were immunostained with anti-active-caspase-3 antibody (positive cells; brown) and counterstained with Hematoxylin (blue). Only a few caspase-3-positive cells (arrowheads) were observed at the base of the crypts in control and DBZ-treated mice. Original magnification 400×. The histogram shows the percentage of crypts containing at least one caspase-3-positive cell (means ± s.e.m. of four mice per condition).
DISCUSSION
This multiparametric approach produced new findings about (1) the cephalo-caudal gene expression (Muc2, Muc5ac and Muc6) of secretory mucins in the mouse gastrointestinal tract (GIT) and (2) the heterogeneity of the effects of γ-secretase inhibition on the secretory lineage differentiation, and on the structural-proliferative units, organization of the stem cell niche and apoptotic compartments of the mouse gut epithelium.
Surprisingly, although the expression of MUC genes is well established in the human GIT (Allen et al., 1998; Van Seuningen et al., 2001), there has been, to our knowledge, no thorough investigation of the expression pattern of these genes in the mouse GIT, except one study at the protein level (Linden et al., 2008). We show here an organ specificity of MUC gene expression along the mouse GIT, with a restricted expression of Muc5ac and Muc6 in the stomach, and of Muc2 in the small intestine and colon with a cephalo-caudal axis gradient. Interestingly, we also found that Math1 expression paralleled that of Muc2, a finding in line with gene targeting studies in mice showing that Math1 drives goblet cell differentiation (Yang et al., 2001; van Es et al., 2010). Then, we used a well-known γ-secretase inhibitor, DBZ, at a dose that was efficient and nontoxic, i.e. without inducing weight loss, neurological signs or diarrhea. Such a concentration of DBZ is effective in blocking the Notch signaling pathway in the small intestine and colon, as demonstrated by the threefold increase in Math1.
We then addressed the issue of whether the GSI DBZ can alter the expression of the mucin genes, quantitatively and qualitatively. We found an overexpression of Muc2 and of Math1 in parallel, all along the intestinal tract (from duodenum to left colon) upon DBZ treatment. Interestingly, ectopic expression of gastric MUC genes has been reported in the human intestine when in stressful situations (Van Seuningen et al., 2001). In addition, as Math1 transfection in some human cancer cell lines strongly enhanced Muc5ac and Muc6 (Sekine et al., 2006), we examined whether overexpression of Muc2 mRNA upon DBZ treatment was accompanied by Muc5ac or Muc6 induction. This is the first demonstration that γ-secretase inhibition quantitatively alters Muc2 expression along the mouse intestinal tract, but does not induce an ectopic expression of Muc5ac or Muc6, and thus does not qualitatively alter MUC genes expression.
Next, we demonstrated, on the basis of morphological analysis of paraffin-embedded ‘swiss rolls’ prepared from the intestinal tracts of DBZ-treated mice, that this Muc2 overexpression is associated with a massive and homogeneous goblet cell conversion, all along the small intestine and colonic crypts. Interestingly, at a high concentration of DBZ, this goblet cell conversion has been reported to be partial and heterogeneous in the intestinal adenomas of ApcMin mice (van Es et al., 2005), suggesting that some neoplastic cells could be resistant to GSI. Altogether, these findings suggest that the early steps of intestinal neoplasia are characterized by the resistance of some intestinal epithelial cells to the effect of GSI on goblet cell conversion, even at high concentrations. Finally, we also showed that DBZ elicits a homogeneous increase in lysozyme-positive Paneth cells in their normal location, the small intestine, a finding in line with reports using Math1-deficient mice, showing that Math1 is required for Paneth cell differentiation (Yang et al., 2001; van Es et al., 2010). Surprisingly, we found that DBZ induces ectopic lysozyme-positive cells in the colon, whereas it does not modify MUC gene organ specificity. These lysozyme-positive cells in the colon could be the ‘intermediate cells’ in transition between Paneth cells and goblet cells, described previously in the mouse intestine (Troughton and Trier, 1969).
It is generally accepted that γ-secretase inhibition, by inducing goblet cell conversion, halts epithelial proliferation in the mouse intestine (van Es et al., 2005; Okamoto et al., 2009), and in the adenomas of ApcMin mice (induction of Math1-positive, PAS-positive, Ki67-negative cells) (van Es et al., 2005). We therefore examined whether the homogeneous conversion of epithelial cells into goblet cells is accompanied by a homogeneous modification of the intestinal structural-proliferative units. We used two complementary methods to address this issue, i.e. an in situ approach and a fractionation of the colonic epithelial cells and subsequent analysis of Ki67-positive cells on cytospin preparations of the different fractions. Both techniques lead to a unique conclusion: DBZ causes an overall decrease in Ki67-positive cells, but this effect on the proliferative compartment is heterogeneous. Indeed, DBZ has three different effects on the colonic crypts: (1) as expected from previous studies (van Es et al., 2005; Okamoto et al., 2009), DBZ (5 μmol/kg) suppresses epithelial cell proliferation, but in only 30% of colonic crypts; (2) DBZ shifted the proliferative compartment upwards in 60% colonic crypts; and (3) DBZ does not alter the location of the proliferative compartment (crypt base) in 10% colonic crypts. Several explanations can be proposed for these findings. Simple ones would be that the dose of DBZ used is insufficient, or that there is a microenvironment-induced restricted access for DBZ. However, these hypotheses can be ruled out because, at the dose used in this study, a homogeneous and massive goblet cell conversion is observed in all crypts along the intestinal tract. A more probable explanation for the heterogeneous effect of DBZ on the proliferative compartment is that some crypts are resistant to γ-secretase inhibition. Hence, our results strongly suggest a differential sensitivity to GSI of intestinal secretory lineage differentiation and crypt cell proliferation state. It is also possible that the effect of a GSI on epithelial proliferation cannot be inferred from its effect on a homogeneous phenotypic, e.g. goblet cell, conversion. In addition, this disturbance of the proliferative compartment might be linked to Math1 overexpression in DBZ-treated mice, because recent experiments in transgenic Math1 mice have shown a reduced and displaced epithelial cell proliferation (VanDussen and Samuelson, 2010).
Another example of the heterogeneous response of colonic crypts to DBZ treatment is in the pattern of CD24 expression in the colon. In fact, CD24 is a marker of cells that are feeders for Lgr5-expressing stem cells in in vitro experiments of intestinal crypt reconstitution (Sato et al., 2011). At the cellular level, CD24 expression is normally restricted to the crypt base, mainly in Paneth cells in the small intestine. In the colon, CD24 is expressed in cells at the crypt base that are equivalent to Paneth cells (Sato et al., 2011). Our findings confirm these data in control mice. Interestingly, we show here that DBZ treatment leads to a heterogeneous expression of CD24 in the colon, some crypts being devoid of CD24. The majority of CD24-negative crypts have lost proliferative cells, as assessed by double immunostaining for CD24 and Ki67. Our findings fit well with the decreased expression of Lgr5, a stem cell marker (Barker et al., 2007), as the mRNA levels were decreased in the F3 fraction of isolated colonic crypts in DBZ-treated mice compared with those of control mice. This downregulation of Lgr5 could hypothetically result from the disappearance of some CD24-positive cells, specific feeder cells for Lgr5 stem cells.
Finally, we examined whether the alterations in the proliferative compartment elicited by DBZ were accompanied by alterations in the apoptotic compartments, i.e. the surface epithelium where differentiated cells are exfoliated, and the base of crypts, in the stem cell compartment (Potten, 1997). Our finding (using cleaved caspase-3 immunohistochemistry, a validated method in the intestine) that DBZ does not modify the number of apoptotic cells, either in the surface epithelium or at the base of crypts, strongly suggests that γ-secretase inhibition does not disturb the apoptotic compartments of the intestinal crypts.
Altogether, these findings point to a differential sensitivity to GSI on goblet cell conversion and reorganization of the intestinal crypt structural-proliferative units, and thus suggest caution for the use of GSIs as chemopreventive agents for intestinal neoplasia.
METHODS
Animal treatment and tissue preparations
C57BL6 female mice (Charles River) that were 9- to 10-weeks old, were maintained under standard conditions, according to the guidelines of the French and local ethical committees. A total of 21 wild-type C57BL6 mice were separated in two groups: (1) four mice were used for analysis of Muc and Math1 mRNA expression along the gastrointestinal tract; (2) 17 mice were used for inhibition of Notch activation. Eight were injected intraperitoneally daily for 8 days consecutively, with either DBZ (5 μmol/kg dissolved in DMSO) and nine with DMSO (control mice). The γ-secretase inhibitor DBZ was custom-synthesized by Syncom (Groningen, Netherlands). Whole body weights of mice were measured every 2 days. Stomach, small and large intestine were removed immediately after the mice were killed by cervical dislocation.
Isolation and fractionation of colonic epithelial cells
Isolation and fractionation of colonic epithelial cells was performed as previously described, with slight modifications (Flandez et al., 2008). Briefly, 35 mm of the right colons were dissected, flushed, everted and filled to distension with PBS before closing the remaining open end. Right colons were washed with shaking in 1× PBS buffer + 1 mM dithiothreitol (DTT) and then transferred into EDTA buffer (1 mM EDTA in PBS + 0.5 mM DTT) and placed on a shaking platform at room temperature. Three fractions of colonic epithelial cells were obtained after three consecutive incubation steps in EDTA buffer (30 minutes each): fraction (F)1 (superficial epithelial cells), F2 (middle of crypts) and F3 (bases of the crypts; Fig. 3C). A few cytospin preparations were performed for Ki67 immunostaining (see below). The remaining cells were harvested by centrifugation and resuspended in lysis buffer for RNA extraction.
Histology, immunohistochemistry, immunocytochemistry and scoring
The stomach as well as the entire small intestine and colon, processed as ‘swiss rolls’ (Moolenbeek and Ruitenberg, 1981), were fixed in 4% formalin. Paraffin sections were stained with Hematoxylin-Eosin and Alcian Blue (for staining of mucins). Immunohistochemistry was performed after antigen retrieval with citrate buffer. Ki67 and CD24 stainings were performed using monoclonal rat anti-mouse antibodies [1:100 (Dako) and 1:500 (eBiosciences), respectively], biotinylated anti-rat antibodies (1:800; Dako) and streptavidin peroxidase (Thermo Scientific). Lysozyme and active-caspase-3 stainings were performed using polyclonal antibodies [1:2000 (Dako) and 1:150 (Cell Signaling), respectively] followed by the Histofine rabbit to mouse kit (Nichirei Biosciences, distributed by Microm-Microtech, France). Diaminobenzidine (DAB) was used as a chromogen. Sections were lightly counterstained with Hematoxylin. In addition, sequential double immunostaining was performed on the same paraffin section for CD24 and Ki67, according to published protocols (Van der Loos, 2008). CD24 was detected as a brown stain using DAB, and Ki67 as a purple stain using the VIP peroxidase substrate kit (Vector Laboratories). To assess the distribution of the proliferative cells along the colonic crypts, a semi-quantitative analysis was performed by counting, on a given section of colon, the number of crypts with proliferative cells in the normal location (crypt base), the number with proliferative cells higher up and those devoid of Ki67-positive cells. At least 150 randomly selected longitudinal sections of crypts were examined. In the small intestine, the number of lysozyme-positive cells per crypt was assessed in 150 randomly selected longitudinal sections of crypts. In the colon, the number of lysozyme-positive cells per 100 crypts was counted. The percentage of active-caspase-3-positive crypts was assessed in the small intestine and colon by counting the crypt bases containing at least one positive cell. At least 500 crypts were examined. Ki67 immunostaining was performed on cytospin preparations of the three fractions of isolated colonic epithelial cells, after acetone fixation and Triton X-100 treatment (0.1% in PBS for 5 minutes). The percentage of Ki67-positive cells was assessed on six randomly selected fields using a 20× objective lens.
RNA isolation and Q-PCR
Total RNA was extracted from the stomach, small intestine (proximal and distal) and colon (right and left) using Tri reagent (Euromedex, Souffelweyersheim, France) and the Fastprep Disrupter (Bio 101, MP Biochemicals, Illkirch, France). For isolated epithelial cells, total RNA was extracted using nucleospin RNA II kit (Macherey-Nagel, Hoerdt, France). Complementary cDNA synthesis was performed as previously described (Jarry et al., 2008). Muc2 (Mm00458299_m1), Muc5ac (Mm01276725_g1), Muc6 (Mm00725165_m1), Math1 (Mm00476035_s1) and β-actin (Mm00607939_s1) mRNAs were quantified using TaqMan Gene Expression Assays and a 7700 thermocycler (Applied Biosystems). Lgr5 mRNA quantification was performed on a Rotorgene 2000 instrument (Corbett Research), using primers for mouse Lgr5, sense 5′-GGGCCTTCAGGTCTTCCTAAAGTCA-3′ and antisense 5′-CCAATGGAATAAAGACGACGGCAACA-3′. PCR amplification was performed using Titanium Taq DNA polymerase (Clontech) as previously described (Jarry et al., 2006). The ratio of each specific transcript to β-actin was calculated.
Statistical analysis
Statistical analyses were performed with GraphPad Prism version 4.0 (GraphPad Software Inc.) using the Mann-Whitney U-test. A P-value less than 0.05 was considered significant.
TRANSLATIONAL IMPACT.
Clinical issue
γ-secretase is a ubiquitous integral membrane multimolecular protease complex. The most well known γ-secretase substrates are amyloid precursor protein (APP) and Notch receptors. Given that aberrant Notch activation has been implicated in various epithelial cancers, including breast cancer, Barrett’s adenocarcinoma of the oesophagus and colon cancer, γ-secretase inhibitors (GSIs; which block the Notch signaling pathway) are a current focus of pharmacological research. The Apcmin mouse model of colon cancer has been validated to assess the chemopreventive and/or chemotherapeutic potential of GSIs in intestinal neoplasia: previous studies indicated that these agents convert proliferating cells into arrested, terminally differentiated goblet cells in intestinal adenomas.
Results
In this study, the authors take a complementary approach to previous studies and address the effects of γ-secretase inhibition in healthy mice, along the full length of the intestinal tract. They focus on identifying the effects of a GSI called dibenzazepine (DBZ) using multiple assays. Their results show that 8 days of DBZ treatment elicits homogenous goblet cell conversion throughout the small intestine and colon, which is associated with a quantitative but not qualitative modulation of the genes encoding secretory mucins (i.e. MUC2 is overexpressed without ectopic expression of gastric MUC5AC and MUC6 genes). Furthermore, DBZ treatment leads to the appearance of ectopic lysozyme-producing cells, so-called ‘intermediate cells’, in the colon. Although the overall effect of DBZ is to suppress intestinal epithelial proliferation, its effects on intestinal crypts are found to be heterogeneous: proliferation is decreased in only 30% of crypts, and the structural-proliferative compartment in most crypts is shifted upwards. Finally, DBZ treatment is found to perturb the stem cell niche in the colon.
Implications and future directions
The heterogenous effects of DBZ on the proliferative compartment suggest that some crypts are resistant to γ-secretase inhibition. Hence, these results suggest that GSIs have different effects on intestinal secretory lineage differentiation and crypt cell proliferation state, and that the effect of these agents on epithelial proliferation cannot be inferred from their ability to induce goblet cell conversion. Therefore, a thorough and multiparametric investigation of the intestinal tract is important for assessing the heterogeneous response to potential therapeutic agents. In addition, the finding that the effects of DBZ on differentiation and proliferation can be uncoupled should encourage caution in administering this drug as a therapy.
Acknowledgments
The authors are grateful to Stéphanie Blandin, Cécile Deleine and Myriam Robard from the Morphology Core Facility (MicroPiCell, IFR26, Nantes), for expert technical assistance. We also thank D. Le Forestier and his staff from the “Photologie” Department for their help.
Footnotes
FUNDING
This work was supported by a fellowship and an award (Prix Alexandre Joël–2009) from ARC (Association pour la Recherche sur le Cancer) to L.D.-D; and in part by the ‘Ligue Contre le Cancer de Loire Atlantique’.
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
A.J., L.D.-D. and C.L.L. conceived and designed the experiments; L.D.-D., M.V., C.B. and A.J. performed the experiments; L.D.-D., M.V., C.B., C.L.L. and A.J. analyzed the data; A.J. and C.L.L. wrote the paper.
REFERENCES
- Al-Hussaini H., Subramanyam D., Reedijk M. J., Sridhar S. S. (2011). Notch signaling pathway as a therapeutic target in breast cancer. Mol. Cancer Ther. 10, 9–15 [DOI] [PubMed] [Google Scholar]
- Allen A., Hutton D. A., Pearson J. P. (1998). The MUC2 gene product: a human intestinal mucin. Int. J. Biochem. Cell Biol. 30, 797–801 [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Rand M. D., Lake R. J. (1999). Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 [DOI] [PubMed] [Google Scholar]
- Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 [DOI] [PubMed] [Google Scholar]
- Flandez M., Guilmeau S., Blache P., Augenlicht L. H. (2008). KLF4 regulation in intestinal epithelial cell maturation. Exp. Cell Res. 314, 3712–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S., Huyghe M., Mourikis P., Robine S., Louvard D., Artavanis-Tsakonas S. (2005). Notch signals control the fate of immature progenitor cells in the intestine. Nature 435, 964–968 [DOI] [PubMed] [Google Scholar]
- Iwatsubo T. (2004). The gamma-secretase complex: machinery for intramembrane proteolysis. Curr. Opin. Neurobiol. 14, 379–383 [DOI] [PubMed] [Google Scholar]
- Jarry A., Bach-Ngohou K., Masson D., Dejoie T., Lehur P. A., Mosnier J. F., Denis M. G., Laboisse C. L. (2006). Human colonic myocytes are involved in postischemic inflammation through ADAM17-dependent TNFalpha production. Br. J. Pharmacol. 147, 64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarry A., Bossard C., Bou-Hanna C., Masson D., Espaze E., Denis M. G., Laboisse C. L. (2008). Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J. Clin. Invest. 118, 1132–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden S. K., Florin T. H., McGuckin M. A. (2008). Mucin dynamics in intestinal bacterial infection. PLoS ONE 3, e3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng R. D., Shelton C. C., Li Y. M., Qin L. X., Notterman D., Paty P. B., Schwartz G. K. (2009). Gamma-secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res. 69, 573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke V., van Es J. H., de Lau W., van den Born M., Kuipers E. J., Siersema P. D., de Bruin R. W., Kusters J. G., Clevers H. (2010). Conversion of metaplastic Barrett’s epithelium into post-mitotic goblet cells by gamma-secretase inhibition. Dis. Model. Mech. 3, 104–110 [DOI] [PubMed] [Google Scholar]
- Milano J., McKay J., Dagenais C., Foster-Brown L., Pognan F., Gadient R., Jacobs R. T., Zacco A., Greenberg B., Ciaccio P. J. (2004). Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 82, 341–358 [DOI] [PubMed] [Google Scholar]
- Moolenbeek C., Ruitenberg E. J. (1981). The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab. Anim. 15, 57–59 [DOI] [PubMed] [Google Scholar]
- Okamoto R., Tsuchiya K., Nemoto Y., Akiyama J., Nakamura T., Kanai T., Watanabe M. (2009). Requirement of Notch activation during regeneration of the intestinal epithelia. Am. J. Physiol. 296, G23–G35 [DOI] [PubMed] [Google Scholar]
- Potten C. S. (1997). Epithelial cell growth and differentiation. II. Intestinal apoptosis. Am. J. Physiol. 273, G253–G257 [DOI] [PubMed] [Google Scholar]
- Real P. J., Tosello V., Palomero T., Castillo M., Hernando E., de Stanchina E., Sulis M. L., Barnes K., Sawai C., Homminga I., et al. (2009). Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukaemia. Nat. Med. 15, 50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., van Es J. H., Snippert H. J., Stange D. E., Vries R. G., Van den Born M., Barker N., Schroyer N. F., van de Wetering M., Clevers H. (2011). Paneth cells constitute the niche for lgr5 stem cells in intestinal crypts. Nature 469, 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine A., Akiyama Y., Yanagihara K., Yuasa Y. (2006). Hath1 up-regulates gastric mucin gene expression in gastric cells. Biochem. Biophys. Res. Commun. 344, 1166–1171 [DOI] [PubMed] [Google Scholar]
- Troughton W. D., Trier J. S. (1969). Paneth and goblet cell renewal in mouse duodenal crypts. J. Cell Biol. 41, 251–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Flier L. G., Clevers H. (2009). Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71, 241–260 [DOI] [PubMed] [Google Scholar]
- Van der Loos C. M. (2008). Multiple immunoenzyme staining: methods and visualizations for the observation with spectral imaging. J. Histochem. Cytochem. 56, 313–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es J. H., van Gijn M. E., Riccio O., van den Born M., Vooijs M., Begthel H., Cozijnsen M., Robine S., Winton D. J., Radtke F., et al. (2005). Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963 [DOI] [PubMed] [Google Scholar]
- van Es J. H., de Geest N., van de Born M., Clevers H., Hassan B. A. (2010). Intestinal stem cells lacking the Math1 tumour suppressor are refractory to Notch inhibitors. Nat. Commun. 1, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Seuningen I., Pigny P., Perrais M., Porchet N., Aubert J. P. (2001). Transcriptional regulation of the 11p15 mucin genes. Towards new biological tools in human therapy, in inflammatory diseases and cancer? Front. Biosci. 6, D1216–D1234 [DOI] [PubMed] [Google Scholar]
- VanDussen K. L., Samuelson L. C. (2010). Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev. Biol. 346, 215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P., Walls M., Qiu M., Ding R., Denlinger R. H., Wong A., Tsaparikos K., Jani J. P., Hosea N., Sands M., et al. (2010). Evaluation of selective gamma-secretase inhibitor PF-03084014 for its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial design. Mol. Cancer Ther. 9, 1618–1628 [DOI] [PubMed] [Google Scholar]
- Yang Q., Bermingham N. A., Finegold M. J., Zoghbi H. Y. (2001). Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155–2158 [DOI] [PubMed] [Google Scholar]