Fig 1.
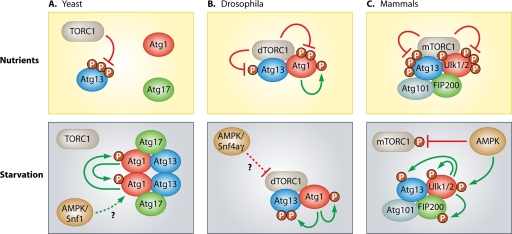
Regulation of autophagy induction by the TOR and AMPK complex in yeast, flies, and mammals. (A) Under nutrient-rich conditions, activated TORC1 inhibits autophagy induction in yeast through direct phosphorylation of Atg13. This hyperphosphorylation of Atg13 prevents binding to Atg1. Inactivation of TORC1, as induced by starvation or rapamycin treatment, results in rapid dephosphorylation of Atg13, leading to the formation of the active Atg1-Atg13-Atg17 kinase complex (50, 51). The Atg13-mediated dimerization of Atg1 has recently been described to be essential for the autophosphorylation and subsequent activation of Atg1 (114). Furthermore, Snf1, a yeast ortholog of mammalian AMPK, has been found to be a positive effector of autophagy, presumably through regulation of Atg1 and Atg13 (107). (B) In contrast to yeast, the Atg1-Atg13 complex is stable under nutrient-rich conditions in Drosophila and dTORC1 phosphorylates both Atg13 and Atg1. In starved animals or when dTORC1 is specifically inhibited, these sites are dephosphorylated, Atg1 kinase activity is elevated, thus leading to autophosphorylation and phosphorylation of Atg13 (10, 11). Snf4aγ, a Drosophila ortholog of the mammalian AMPK gamma subunit, was found to be an inducer of autophagy; nevertheless, the exact mechanism remains elusive (68). (C) In mammals, two orthologs of yeast Atg1, termed Ulk1 and Ulk2, have been linked to starvation induced autophagy. Both are found in a stable complex with Atg13, FIP200 (5, 26, 28, 29, 37, 47), and Atg101, an additional binding partner of Atg13 that has no ortholog in yeast (38, 78). In contrast to yeast, the composition of this complex does not change with the nutrient status. Under fed conditions mTORC1 phosphorylates Ulk1/2 and Atg13, thereby inhibiting the kinase complex (26, 37, 47). In response to starvation, the mTORC1-dependent phosphorylation sites in Ulk1/2 are rapidly dephosphorylated, and Ulk1/2 autophosphorylates and phosphorylates Atg13 and FIP200. Alternatively, Ulk1/2 is phosphorylated by AMPK and thereby activated (20, 54). In addition, AMPK indirectly leads to the induction of autophagy by inhibiting mTORC1 through phosphorylation of raptor (27, 64). Note, however, that this is a schematic overview, and we do not provide any determination regarding the association between AMPK and the Ulk1/2-Atg13-FIP200 complex due to the conflicting results published in the recent past. For further details, see the legend to Fig. 3. (This figure was adopted and modified with permission from reference 11.)