Fig 2.
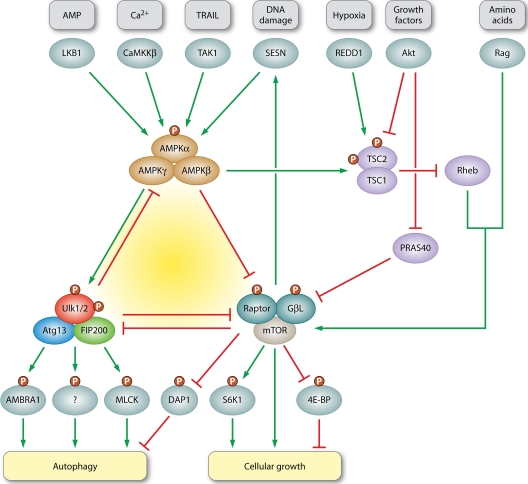
Fine adjustment of autophagy by the AMPK-mTORC1-Ulk1/2 kinase network. The two protein complexes AMPK and mTORC1 are known to oppositely regulate the autophagy inducing complex Ulk1/2-Atg13-FIP200. Under sufficient supply of growth factors and nutrients, the active mTORC1 stimulates growth related processes such as protein translation, e.g., by phosphorylation of S6K1 and 4E-BP, while simultaneously inhibiting self-consuming processes such as autophagy (94, 110). mTORC1 activity is positively regulated by growth factor signaling via the PI3K-Akt pathway. Akt activates mTORC1 by inhibition of TSC1/2 (41, 71, 72, 119) or PRAS40 (105), two negative regulators of mTORC1 activity that both antagonize the Rheb-mediated activation. Hypoxia counteracts mTORC1 activation via the TSC1/2-Rheb pathway, e.g., by upregulation of REDD1 (7). Amino acids, in contrast, stimulate the Rag-GTPase-dependent recruitment of mTORC1 to lysosomes and its subsequent activation by Rheb-GTPases (54, 89, 90). The catalytic activity of AMPK crucially depends on phosphorylation by upstream kinases, such as the constitutively active LKB1. AMPK activity is further enhanced by decreasing ATP/AMP ratios (30). In addition, the other two known upstream kinases, CaMKKβ and TAK1, have been implicated in AMPK-mediated autophagy induction by intracellular [Ca2+] and TRAIL treatment, respectively (36, 39). Under low-energy conditions, AMPK positively regulates autophagy induction (77) through inhibition of mTORC1. This releases the negative regulation of mTORC1 on the Ulk1/2-Atg13-FIP200 complex, especially on Ulk1/2 kinase activity (26, 37, 47). AMPK inhibits mTORC1 either via the TSC1/2-Rheb pathway (44) or by direct phosphorylation of raptor (27). However, AMPK is also able to bind, phosphorylate, and directly activate Ulk1/2 (5, 21, 55, 64, 88, 95). Again, this interaction is counteracted by mTORC1 (55). For a more detailed discussion see Roach (88) and Fig. 3. Prolonged TORC1 activation, on the other hand, leads to the accumulation of Sestrin (SESN) in Drosophila, a DNA damage-inducible protein that suppresses TOR activity by AMPK activation (63). Furthermore, mTORC1 not only inhibits autophagy by suppressing Ulk1/2 kinase activity, it also simultaneously inhibits DAP1, a negative regulator of autophagy (57). mTORC1 inhibition thus leads to both autophagy induction via Ulk1/2-Atg13-FIP200 and to its restriction via DAP1. Ulk1 kinase activity might be linked to autophagy induction in several ways. Two downstream targets of Ulk1 have been proposed thus far. First, Ulk1 directly phosphorylates AMBRA1, a Beclin1-interacting protein and regulatory component of the PI3K class III complex (17) and, second, it phosphorylates and activates a distinct myosin light chain kinase (MLCK) in mammals (ZIPK) and Drosophila (Sqa) (100). Two Ulk1-dependent feedback loops additionally help to fine-tune the autophagic response. Ulk1 has been shown to phosphorylate and inhibit both of its upstream regulators AMPK and mTORC1. While phosphorylation of raptor might help to maintain mTORC1 inhibition when nutrients are limited (19, 48), the inhibition of AMPK activity by Ulk1 antagonizes this action and restricts the autophagic response (70). This perplexingly complex network of mutual activation and inhibition will ultimately establish an appropriate response to conflicting demands.