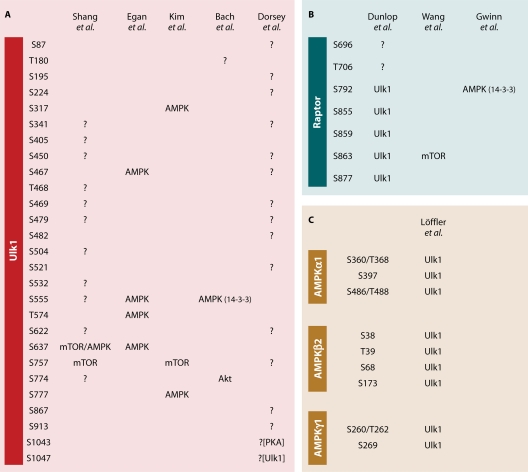
Fig 3.
Mutual phosphorylation of Ulk1, mTORC1, and AMPK. (A) Ulk1 is a hyperphosphorylated protein that is massively dephosphorylated upon starvation. Under normal growth conditions, mTORC1 has been shown to directly bind to and negatively regulate Ulk1/2 kinase activity by direct phosphorylation (26, 37, 47). A total of 16 phosphorylation sites in mouse Ulk1, purified from HEK293T cells under fed conditions, were first mapped by Dorsey et al. (18). These authors additionally identified two sites, differentially phosphorylated in wild-type versus kinase-dead Ulk1 (S1043 and S1047). While the one was suggested as direct target for autophosphorylation, the other might represent a putative PKA site (18). Shang et al. (95) quantitatively analyzed the differential phosphorylation status in human Ulk1 purified from HEK293T cells between fed versus starved conditions (HBSS containing 1% rich medium) using stable isotope labeling with amino acids in cell culture (SILAC). A total of 13 sites were identified, with the strongest dephosphorylation at S638 and S758 (corresponding to S637 and S757 in mouse Ulk1) showing a >10-fold decrease after starvation, although with different kinetics. The same decrease was seen after rapamycin treatment and mTOR knockdown (95). Interestingly, phosphorylation at S638 was affected by the knockdown of AMPKα and AMPKβ (95). Shang et al. found that AMPK was associated with Ulk1 under fed conditions and proposed that the dephosphorylation of S758 seen after starvation is critical for the dissociation of AMPK/Ulk1. In contrast, Lee et al. (64) proposed that the interaction between AMPK and Ulk1 is essential for the induction of autophagy and that AMPK activity both recruits 14-3-3 proteins to the complex and leads to inactivation of mTORC1 activity by AMPK-mediated phosphorylation of raptor at S792 (27, 64). Egan et al. (21) additionally identified Ulk1 both as an AMPK substrate and as a 14-3-3 binding protein. This group found S467, S555, T574, and S637 of Ulk1 to be phosphorylated after phenformin treatment and confirmed these sites in an AMPK in vitro kinase assay (21). Notably, Bach et al. (4) could meanwhile confirm the AMPK-dependent phosphorylation at S555 and that this induces the binding to 14-3-3 proteins. This group additionally identified a critical phosphorylation site in the Ulk1 activation loop (T180), as well as a potential Akt phosphorylation site (S774) whose phosphorylation is increased after insulin treatment. The phosphorylation and differential regulation of Ulk1 by mTORC1 and AMPK has also been reported by Kim et al. (55). This group identified S757 in mouse Ulk1 as a direct mTOR site, the same identified by Shang et al. in human Ulk1 (95). However, Kim et al., in contrast, suggest that phosphorylation at S757 prevents the interaction between Ulk1 and AMPK. mTORC1 inhibition would thus lead to an association of AMPK and Ulk1. In line with that, the data suggest an activating effect of AMPK on Ulk1 kinase activity by direct phosphorylation at S317 and S777. These authors found that both sites are required for Ulk1 activation after glucose starvation (55). Notably, neither of these two sites has been identified by one of the other groups. All phosphorylation sites identified by the five groups (4, 18, 21, 55, 95) are shown in single-letter code and refer to mouse Ulk1. Proposed kinases are either indicated by name or otherwise labeled with question marks (?). (B) Dunlop et al. (19) and Jung et al. (48) identified raptor as a direct substrate of Ulk1 and Ulk1 thereby as a negative regulator of either mTORC1 activity (48) or substrate binding (19). After overexpression of Ulk1, Dunlop et al. observed an increase in the in vivo phosphorylation of raptor at S696, T706, S792, S855, S859, and S863 using phospho-specific antibodies and could subsequently confirm the latter four sites as directly phosphorylated by Ulk1 (19). The strongest phosphorylation was seen on S859. Interestingly, S792 is the AMPK and 14-3-3 binding site identified by Gwinn et al. (27), by which AMPK negatively regulates mTORC1 activity, while S863 is known to be phosphorylated by mTOR and to promote mTORC1 activity (23, 106). (C) Löffler et al. (70) identified all three subunits of AMPK as a direct substrate of Ulk1 and Ulk2 and mapped several Ulk1-dependent in vitro phosphorylation sites in AMPKα1, -β2, and -γ1 (residues refer to rat AMPK, for some peptides the phospho-acceptor sites could not be distinguished: S360/T368, S486/T488, and S260/T262). The Ulk1-dependent phosphorylation of AMPK has been proposed to negatively regulate AMPK kinase activity, thus constituting a negative regulatory feedback loop (70). (This figure was adopted and modified with permission from reference 88.)