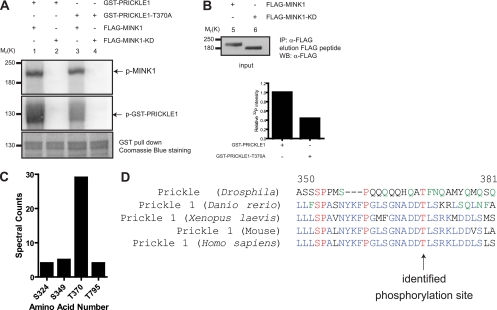
Fig 3.
PRICKLE1 is a substrate for the serine/threonine kinase MINK1. (A) Recombinant GST-PRICKLE1 and GST-PRICKLE1 (T370A) were used as substrates for in vitro phosphorylation assays using purified MINK1 or MINK1-KD. The top panel (lanes 1 to 4) shows the autophosphorylation of MINK1 bound to recombinant PRICKLE1 and also indicates that equal amounts of kinase were used. In the middle panel, recombinant PRICKLE1 is phosphorylated by MINK1 (lane 1) but not by MINK1-KD (lane 2). The phosphorylation of PRICKLE1 (T370A) is dramatically reduced (lane 3). The bottom panel represents the loading of PRICKLE1 and PRICKLE (T370A) assessed using Coomassie blue staining. The input of purified MINK1 and MINK1-KD is shown in lanes 5 and 6 using Western blotting. (B) Quantification of PRICKLE1 phosphorylation by MINK1. The mutation of threonine 370 to alanine inhibits Pk phosphorylation by 57%. (C) Mass spectrometry analysis of the in vitro kinase reactions revealed four potential phosphorylation sites in PRICKLE1. Spectral counts represent the total number of independent spectra assigned to the indicated phosphopeptides. A total number of 1,819 peptides were surveyed for PRICKLE1 in three independent experiments. (D) Alignments of human PRICKLE1 with the mouse, Xenopus laevis, Danio rerio, and Drosophila homologues show that T370 is evolutionarily conserved.