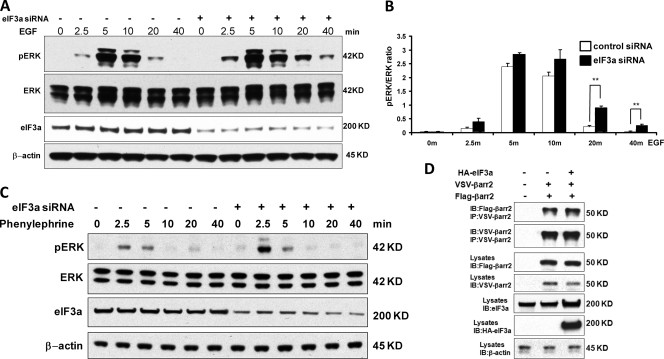
Fig 3.
Knockdown of eIF3a causes sustained ERK activity in response to EGF. (A) eIF3a was knocked down in HEK293 cells with siRNA, and knockdown efficiency was monitored by Western blotting with β-actin as a loading control. Cells were treated with EGF for the indicated time points, and ERK activation was examined by blotting with a phospho-specific antibody. (B) The ratio of phospho-ERK to total ERK (pERK/ERK) was quantified by densitometric evaluation of Western blots. The data represent the means ± the SEM of six independent experiments. ∗∗, P < 0.01. (C) eIF3a was knocked down in HEK293 cells with siRNA, and knockdown efficiency was monitored by Western blotting. Serum-starved cells were treated with the a1B-adrenergic receptor agonist phenylephrine (10−4 M) for the indicated time points, and phospho-ERK was detected by Western blotting. The same membrane was reprobed for total ERK and β-actin as loading controls. (D) Overexpression of eIF3a does not affect β-arrestin2 dimerization. HEK293 cells were cotransfected with VSV-β-arrestin2, Flag-β-arrestin2 and HA-eIF3a as indicated. The corresponding empty vectors were used as a control (in the lanes labeled “–”). At 24 h after transfection, VSV-β-arrestin2 was immunoprecipitated and blotted with antibodies against the VSV and Flag tags. The expression of transfected proteins was monitored by blotting cell lysates. β-Actin was used as loading control.