Abstract
Because of the tradition of using honey as an antimicrobial medicament, we investigated the effect of natural honey (NH) on Streptococcus mutans growth, viability, and biofilm formation compared to that of an artificial honey (AH). AH contained the sugars at the concentrations reported for NH. NH and AH concentrations were obtained by serial dilution with tryptic soy broth (TSB). Several concentrations of NH and AH were tested for inhibition of bacterial growth, viability, and biofilm formation after inoculation with S. mutans UA159 in 96-well microtiter plates to obtain absorbance and CFU values. Overall, NH supported significantly less (P < 0.05) bacterial growth than AH at 25 and 12.5% concentrations. At 50 and 25% concentrations, both honey groups provided significantly less bacterial growth and biofilm formation than the TSB control. For bacterial viability, the results for all honey concentrations except 50% NH were not significantly different from those for the TSB control. NH was able to decrease the maximum velocity of S. mutans growth compared to AH. In summary, NH demonstrated more inhibition of bacterial growth, viability, and biofilm formation than AH. This study highlights the potential antibacterial properties of NH and could suggest that the antimicrobial mechanism of NH is not solely due to its high sugar content.
INTRODUCTION
Honey has been used as a source of nutrients as well as a medicine since ancient times (3). Recent publications indicating the effect of honey in the management of certain conditions have rekindled interest in honey as a natural therapeutic agent. For example, honey can be used as a temporary dressing in burns (20). It has also been found to be effective in the management of radiation-induced mucositis in patients receiving head and neck radiotherapy (26). In addition, Robson and colleagues recorded accelerated healing when honey was used as a wound dressing material in a randomized clinical trial (28).
The antibacterial properties of honey have been well documented (37). However, the specific antimicrobial mechanism of honey is still unclear (7). Among the possible mechanisms are the presence of inhibitory factors such as flavonoids (16) and hydrogen peroxide (35, 36), low pH (38), and high osmolarity due to its sugar concentration (38).
Honey may have a similar antibacterial effect on Streptococcus mutans, which is considered the main causative organism of dental caries (21). S. mutans along with other oral bacteria forms on the tooth surface a microbial community surrounded by extracellular matrix and salivary proteins (22), collectively known as dental biofilm. Cariogenic bacteria within this biofilm utilize dietary sugars and produce lactic acid as a by-product (34). This acid attacks and demineralizes the tooth structure, leading to decay.
Very limited studies have investigated the effect of honey on S. mutans. These studies investigated the effect of honey on several strains of oral bacteria (7). Here, we tried to explore the effect of honey on the growth and viability of S. mutans, as well as determine the effect of natural honey on S. mutans biofilm formation.
Many agents have been considered in the goal of preventing dental caries, including chlorhexidine (4), fluoride (12), and xylitol (27). However, the dietary effect of ingestible carbohydrate sources cannot be ignored. Honey is sometimes used as a sugar substitute to limit the exposure to sucrose (31). The effect of honey on S. mutans biofilms can provide evidence on both the cariogenicity and antibacterial properties of honey. In this study, we investigated the effect of honey on the growth, viability, and biofilm formation of S. mutans.
MATERIALS AND METHODS
Bacterial preparation and reagents.
S. mutans UA159 (1) was isolated from dental plaque above a carious enamel surface. S. mutans UA159 was transferred from an agar plate into a sterile tube containing tryptic soy broth (TSB; Difco Laboratories, Detroit, MI) and incubated for 24 h in a 5% CO2 incubator at 37°C. Natural honey (NH) was bought from a local grocery store in Jeddah, Saudi Arabia (Langnese Honig, Germany). Artificial honey (AH) was prepared as described by Wilkinson and Cavanagh (37). AH ingredients, including 40.5% fructose, 33.5% glucose, 7.5% maltose, and 1.5% sucrose, were added incrementally and stirred until dissolved in deionized water. AH was diluted 1:2 (5 ml AH plus 5 ml of TSB) and then filter sterilized through a 45-μm-pore-size filter and stored at 4°C until needed.
Biofilm assay.
Different NH and AH concentrations were obtained by serial dilution with TSB. Sterile 96-well flat-bottom polystyrene microtiter plates (Fisher Scientific, Pittsburgh, PA) were utilized. Wells containing bacteria received 290 μl of TSB-honey dilutions and were inoculated with 10 μl of S. mutans from the overnight culture. Wells without bacteria received 300 μl of the TSB-honey dilution. The microtiter plates were incubated for 24 h at 37°C in 5% CO2 without agitation.
Determination of bacterial growth and biofilm formation.
The absorbance of each well in the microtiter plates was read at 540 nm in a microplate spectrophotometer (Molecular Devices, Inc., Sunnyvale, CA) to determine the amount of bacterial growth (planktonic and biofilm bacteria). In order to determine the effect of honey on biofilm formation, the planktonic bacteria were removed by pipetting. The biofilm in the microtiter plates was fixed by adding 100 μl of 10% formaldehyde solution and left overnight at room temperature. The formaldehyde was removed from the wells, 100 μl of 0.1% crystal violet was added, and the plates were kept at room temperature for 1 h. The crystal violet solution was removed, and 250 μl of isopropanol was placed in each well to release the crystal violet and then aspirated to manually mix the contents of the wells. The absorbance of each well was read at 490 nm.
Planktonic growth curves were obtained by placing 190 μl of honey at each concentration in TSB into a 96-well microtiter plate. Wells with corresponding concentrations of sucrose in TSB (TSBS) were also included. The wells were inoculated with 10 μl of an overnight S. mutans culture in triplicate. Bacterial growth curves were recorded by a microtiter plate reader at 595 nm and 37°C every 20 min for 20 h.
Determination of bacterial viability.
In order to assess the effect of honey on S. mutans viability, aliquots of bacterial cultures from the 50, 25, 12.5, and 6.25% wells of both honey groups were diluted 1:1,000 and spiral plated on blood agar plates in duplicate, and the plates were incubated for 48 h in a 5% CO2 incubator at 37°C. The number of CFU for each concentration of honey was determined using an automated colony counter (Synbiosis, Inc., Frederick, MD) and compared to values from the TSB control culture. This experiment was repeated, and combined results were reported.
To investigate the effect of NH and AH on viable sessile cells, a sodium 3′-[1-[(phenylamino)-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene-sulfonic acid hydrate (XTT) assay was utilized (29). Biofilms were allowed to form in a microtiter plate overnight by adding 10 μl of an overnight culture of S. mutans to 290 μl of 1% TSBS and incubating in 5% CO2 at 37°C. Biofilms were washed three times with sterile 0.9% NaCl to remove nonadherent cells. NH, AH, and TSBS concentrations (containing 1.56% through 50% sucrose) were added to designated wells (in triplicate), and the plates were incubated at 37°C for 24 h. After incubation, the treated biofilms were washed three times and the metabolic activity of the biofilms was determined by the addition of XTT. After 2 h of incubation at room temperature in the dark, the absorbance at 490 nm was determined.
Statistical analysis.
Statistical testing was conducted using a SigmaStat (version 11.0) statistical package. For bacterial growth, biofilm formation, and XTT assays, one-way analysis of variance followed by the Holm-Sidak pairwise comparison method was used to compare the results for NH, AH, and the TSB (in addition to TSBS in the XTT assay) control for each concentration tested. Student's t test was used to assess bacterial viability at different AH and NH concentrations. A 0.05 statistical significance level was used for all statistical tests.
RESULTS
The cultures in the wells of AH at 50, 25, and 12.5% concentrations were more turbid than the respective NH wells, indicating more bacterial growth (Fig. 1). However, this observation was based on visual comparison between AH and NH wells from each microtiter plate experiment. The remaining wells were visually similar between AH and NH wells. In addition, during the fixation process, the biofilms in the NH wells were easily detached from the base of the wells. On the other hand, biofilms in the AH wells were more tightly bound to the microplate wells and were less disturbed by the staining process.
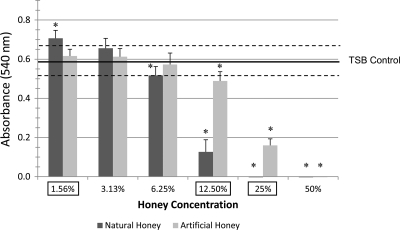
Fig 1.
S. mutans planktonic and biofilm cell growth absorbance values for NH and AH concentrations. The absorbance values of the 50% NH- and 50% AH-treated cultures as well as the 25% NH-treated culture were below 0.001. Horizontal lines, means and SEs of the TSB control; error bars, SEs; asterisks, significant differences (P ≤ 0.05) from the TSB control; boxed concentrations, significant difference between NH and AH groups for a specific concentration.
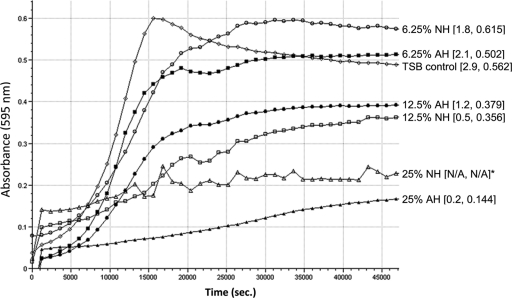
Overall, NH wells demonstrated significantly less bacterial growth at 50, 25, 12.5, and 6.25% concentrations than the TSB control wells and less growth at 25 and 12.5% than the AH wells (Fig. 1). Both honey groups exhibited bacterial growth values equal to or higher than those for the TSB control at the 3.13 and 1.56% concentrations. NH inhibited the maximum velocity of growth considerably more than AH (Fig. 2). In addition, the overall maximum absorbance values were lower in the NH groups than their AH counterparts.
Fig 2.
S. mutans growth curves for NH and AH concentrations compared to the TSB control. The graph shows absorbance values over a period of 12.5 h (45,000 s). Numbers in brackets next to groups indicate mean Vmax and mean maximum absorbance, respectively. An asterisk indicates that Vmax and maximum absorbance could not be calculated due to the influence of the high absorbance values of NH at 25% (N/A, not available). Note that there was no bacterial growth at this concentration.
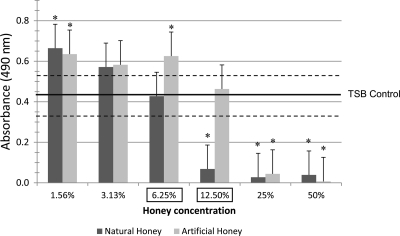
For biofilm formation data, the values for NH were statistically lower than those for the TSB control at concentrations greater than 6.25% (Fig. 3). On the other hand, values for AH wells were always higher than those for the TSB control wells, except at the 50 and 25% concentrations. Compared to AH, NH demonstrated significantly less biofilm formation at 12.5 and 6.25%.
Fig 3.
S. mutans biofilm formation absorbance values for NH and AH concentrations after staining with crystal violet. Horizontal lines, means and SEs of the TSB control; error bars, SEs; asterisks, significant differences (P ≤ 0.05) from TSB control; boxed concentrations, significant difference between NH and AH groups for a specific concentration.
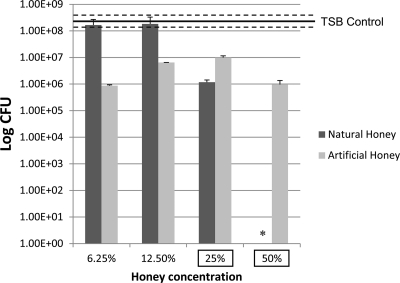
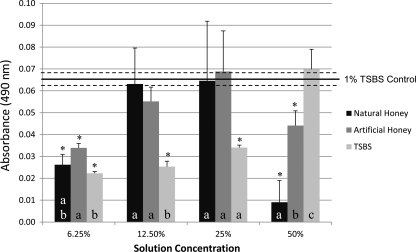
Regarding bacterial viability, the results for all honey concentrations except 50% NH were not significantly different from those for the TSB control (Fig. 4). Significant differences between NH and AH at 50 and 25% concentrations were found. Data from the XTT assay followed a similar trend (Fig. 5). NH at 50% was able to decrease the amount of viable sessile cells compared to AH and TSBS at the same concentration. Results for NH and AH at 25, 12.5, and 6.25% were not statistically different; however, both of their values at 6.25% were statistically lower than those for the 1% TSBS control.
Fig 4.
S. mutans viability presented as numbers of CFU in a logarithmic scale of selected concentrations of NH and AH. The viability of the 50% NH-treated sample was almost zero. Horizontal lines, means and SEs of the TSB control; error bars, SEs; asterisks, significant differences (P ≤ 0.05) from the TSB control; boxed concentrations, significant difference between NH and AH groups for a specific concentration.
Fig 5.
S. mutans XTT viability assay absorbance values for NH, AH, and TSBS. Horizontal lines, means and SEs of the 1% TSBS control; error bars, SEs; asterisks, significant differences (P ≤ 0.05) from the 1% TSBS control. Groups with different letters were significantly different (P ≤ 0.05). Comparisons were made between solutions with the same concentration.
DISCUSSION
Several reports demonstrating the effectiveness of honey in the treatment of ulcers and infected wounds can be found in the literature (10, 20, 25, 39). Honey has also been found to be effective in treating oral conditions, such as ulcers, mucositis, and periodontal disease (8, 11, 26). This effect is largely attributed to the antibacterial properties of honey. However, the exact antimicrobial mechanism of honey has not been determined yet.
Honey is used as a natural sweetener and could potentially promote dental caries, although conflicting data regarding honey's cariogenicity have been reported (24). Honey has high concentrations of sugars (about 70%); (31) despite this, many types of honey have antibacterial properties (2, 38). However, could the antibacterial activity of honey counteract its cariogenic potential?
The literature describes very limited studies that have investigated the effect of honey on S. mutans. In this study, we investigated the effect of NH on S. mutans growth, biofilm formation, as well as viability. We have considered one of the proposed antimicrobial mechanisms of honey, which is its high osmolarity due to an intense sugar content. Therefore, we prepared an AH solution that contained sugar concentrations comparable to those found in NH on the basis of previous reports (36a, 37).
Overall, NH inhibited the growth and biofilm formation of S. mutans at concentrations between 50 and 12.5%. The AH solution did not demonstrate a similar effect. At 12.5%, there was a clear difference between NH and AH in the inhibitory effect on growth and biofilm formation. Although the MIC of honey has been reported to range from 50 to 0.25% according to the type of honey (23), the honey used in this study inhibited S. mutans at concentrations of between 25 and 12.5%. This range fits the data generated by Basson et al., who found the MIC for S. mutans to be about 21% when they tested the effect of NH on several oral bacteria (7). In a recent study, two types of manuka honey were able to reduce the adherence of S. mutans to glass surfaces at concentrations above 200 μg/ml (5). The authors found a clear difference between the two tested honey samples and suggested that different types of honey may have different antibacterial potentials. The same hypothesis has been stated in other reports (2, 11, 38). In the current study, we found visual evidence that biofilms in the NH groups were loosely bound to the wells. This could suggest that the expression of molecules needed for S. mutans adherence, such as antigen I/II, might be altered by NH. In another study, plaque levels and bleeding scores were reduced in participants who chewed honey leather compared to controls using chewing gum (11).
At lower concentrations (≤6.25%), both NH and AH exhibited results comparable to those for the TSB control. This could indicate that the active ingredient in honey was diluted to the degree that renders it ineffective. A similar effect has been reported previously (33). In that study, honey at 4.16% increased the growth of S. mutans and S. sobrinus; however, there was a decrease in growth at higher concentrations, with total inhibition occurring at 25%. The difference in the bacterial and biofilm growth values between the NH and AH groups could indicate that the high sugar concentration was not the decisive factor, since the sugar contents in the AH and NH groups were comparable. This concept has been questioned by Steinberg and colleagues in their above-mentioned study (33). Although the artificial honey solution that they used is comparable to the one used in this study (37.5% fructose, 30.5% glucose, and 1.5% sucrose), the clinical design of their study could have contributed to their findings. Despite that, there was a clear difference between NH and AH in bacterial growth of S. sobrinus in their study. Growth curve results indicated that NH was able to decrease the maximum velocity (Vmax) of S. mutans growth to a greater degree than AH.
Results from viability tests were less conclusive. No differences were detected between NH and AH concentrations when compared to the TSB control except for 50% NH. Although NH and AH displayed an inhibitory effect on bacterial growth in the assay, we still found some growth when we cultured the aliquots on blood agar. One reason for this could be the lower sensitivity of the bacterial growth assay. That is why we found very low absorbance values for the 50 and 25% concentrations in the growth assay but observed some growth during culturing on the blood agar. Data from the XTT assay confirmed the viability test results, with NH and AH at 50, 25, and 12.5% behaving in the same manner in both assays. The only difference was at 6.25%, which could be related to differences between planktonic and sessile cell susceptibility to honey.
Initially, high absorbance values were recorded for the NH group at the higher concentrations (50 through 12.5%) in the growth assay. This led us to suspect that these values could be due to the presence of honey itself and not due to actual bacterial growth. In order to investigate this, we included diluted NH and AH wells at the same concentrations tested but without bacterial inoculation. Absorbance values from these wells were subtracted from readings of their corresponding wells that were inoculated with S. mutans. As we have assumed, the presence of NH initially altered the absorbance values obtained. This effect was more pronounced at the higher honey concentrations and was decreased for the more diluted solutions. This effect was also found in the AH groups; however, the changes in the absorbance values were negligible.
The mechanism of the antibacterial effect of honey is not fully understood; however, several proposed mechanisms have been mentioned in the literature. Hydrogen peroxide, a potent antimicrobial agent, is produced in honey by the action of the glucose oxidase enzyme (23, 36). However, according to Barnard and Stinson, the alpha-hemolysin produced by the viridians group streptococci, including S. mutans, is hydrogen peroxide (6), and this could preclude the suggestion that hydrogen peroxide is a major antimicrobial mechanism of honey against S. mutans. Flavonoids, which are a group of pigments produced by plants, have also been found in honey. Their presence was suggested to be a potential cause for the antimicrobial properties of honey (16). Another compound that was reported to have an antibacterial property is methylglyoxal (18). This compound is present in manuka honey and could be responsible for its antibacterial behavior. However, after inactivation of this molecule, honey samples retained their antimicrobial properties (19). An alternative possible explanation for the phenomenon is the high sugar concentration of NH. According to previous reports, the total amount of sugars in NH is between 70 and 80% (31, 32). This high sugar load causes hypertonic conditions that lead to lysis of microbial cell walls (17). However, results from the present study challenge this theory.
Despite the inhibitory effect of honey on S. mutans, this effect may be different in the oral cavity. The cariogenic potential of the sugary constituents of honey is a subject of debate, and evidence based on previous reports is inconclusive. In animal models, comparable cariogenic activity was reported for glucose and fructose, the main sugars of honey, compared to sucrose (14, 15). In a recent study, rats were fed different fluids to test their cariogenicity (9). Although honey promoted the development of caries lesions on smooth surfaces of teeth, values from the honey group were statistically lower than those observed in the sucrose group. On the other hand, some reports indicate that honey sugars have fewer cariogenic properties than sucrose. Frostell and colleagues reported a lower caries incidence in hamsters and rats when they substituted sucrose for a mixture of glucose, fructose, and maltose (13). In another study, authors have reported a lower caries incidence (represented by lower decayed, missed, and filled per tooth surface [DMFS] values) for fructose than sucrose in a 2-year clinical trial (30).
In summary, the following could be concluded from this study: (i) there was a difference between the effect of NH and that of AH; (ii) at 12.5%, NH supported less bacterial growth and biofilm formation than AH, which contained the same amount of sugars; (iii) the sugar content might not be the only decisive factor for the antibacterial property of honey; (iv) the suggested MIC for natural honey is between 25 and 12.5%; (v) NH was able to decrease the Vmax of S. mutans growth compared to AH; and (vi) further studies are needed to identify the antimicrobial mechanism of NH.
ACKNOWLEDGMENTS
Hani M. Nassar was sponsored by a scholarship from King Abdulaziz University, Jeddah, Saudi Arabia. This work was supported by the Ph.D. Program in Dental Science of the Indiana University School of Dentistry.
Footnotes
Published ahead of print 28 October 2011
REFERENCES
- 1. Ajdić D, et al. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. U. S. A. 99:14434–14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen KL, Molan PC, Reid GM. 1991. A survey of the antibacterial activity of some New Zealand honeys. J. Pharm. Pharmacol. 43:817–822 [DOI] [PubMed] [Google Scholar]
- 3. Aristotle 1910 (350 BC). Historia animalium. In Smith JA, Ross WD. (ed), The works of Aristotle. Oxford University, Oxford, United Kingdom: (Translated by Thompson DAW.) [Google Scholar]
- 4. Autio-Gold J. 2008. The role of chlorhexidine in caries prevention. Oper. Dent. 33:710–716 [DOI] [PubMed] [Google Scholar]
- 5. Badet C, Quero F. 2011. The in vitro effect of manuka honeys on growth and adherence of oral bacteria. Anaerobe 17:19–22 [DOI] [PubMed] [Google Scholar]
- 6. Barnard JP, Stinson MW. 1996. The alpha-hemolysin of Streptococcus gordonii is hydrogen peroxide. Infect. Immun. 64:3853–3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Basson NJ, du Toit IJ, Grobler SR. 1994. Antibacterial action of honey on oral streptococci. J. Dent. Assoc. S. Afr. 49:339–341 [PubMed] [Google Scholar]
- 8. Biswal BM, Zakaria A, Ahmad NM. 2003. Topical application of honey in the management of radiation mucositis: a preliminary study. Support. Care Cancer 11:242–248 [DOI] [PubMed] [Google Scholar]
- 9. Bowen WH, Lawrence RA. 2005. Comparison of the cariogenicity of cola, honey, cow milk, human milk, and sucrose. Pediatrics 116:921–926 [DOI] [PubMed] [Google Scholar]
- 10. Cooper RA, Molan PC, Harding KG. 1999. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J. R. Soc. Med. 92:283–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. English HK, Pack AR, Molan PC. 2004. The effects of manuka honey on plaque and gingivitis: a pilot study. J. Int. Acad. Periodontol. 6:63–67 [PubMed] [Google Scholar]
- 12. Fejerskov O, Thylstrup A, Larsen MJ. 1981. Rational use of fluorides in caries prevention. A concept based on possible cariostatic mechanisms. Acta Odontol. Scand. 39:241–249 [DOI] [PubMed] [Google Scholar]
- 13. Frostell G, Keyes PH, Larson RH. 1967. Effect of various sugars and sugar substitutes on dental caries in hamsters and rats. J. Nutr. 93:65–76 [DOI] [PubMed] [Google Scholar]
- 14. Green RM, Hartles RL. 1969. The effect of diets containing different mono- and disaccharides on the incidence of dental caries in the albino rat. Arch. Oral Biol. 14:235–241 [DOI] [PubMed] [Google Scholar]
- 15. Grenby TH, Hutchinson JB. 1969. The effects of diets containing sucrose, glucose or fructose on experimental dental caries in two strains of rats. Arch. Oral Biol. 14:373–380 [DOI] [PubMed] [Google Scholar]
- 16. Havsteen B. 1983. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharmacol. 32:1141–1148 [DOI] [PubMed] [Google Scholar]
- 17. Jay J. 1970. Modern food microbiology. Van Nostrand Reinhold Company, New York, NY [Google Scholar]
- 18. Kwakman PH, te Velde AA, de Boer L, Speijer D, Vandenbroucke-Grauls CM, Zaat SA. 2010. How honey kills bacteria. FASEB J. 24:2576–2582 [DOI] [PubMed] [Google Scholar]
- 19. Kwakman PH, Te Velde AA, de Boer L, Vandenbroucke-Grauls CM, Zaat SA. 2011. Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS One 6:e17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lineen E, Namias N. 2008. Biologic dressing in burns. J. Craniofac. Surg. 19:923–928 [DOI] [PubMed] [Google Scholar]
- 21. Loesche WJ. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marsh PD. 2004. Dental plaque as a microbial biofilm. Caries Res. 38:204–211 [DOI] [PubMed] [Google Scholar]
- 23. Molan PC. 1992. The antibacterial activity of honey: 2. Variation in the potency of the antibacterial activity. Bee World 73:59–76. [Google Scholar]
- 24. Molan PC. 2001. The potential of honey to promote oral wellness. Gen. Dent. 49:584–589 [PubMed] [Google Scholar]
- 25. Molan PC. 2002. Re-introducing honey in the management of wounds and ulcers—theory and practice. Ostomy Wound Manage. 48:28–40 [PubMed] [Google Scholar]
- 26. Motallebnejad M, Akram S, Moghadamnia A, Moulana Z, Omidi S. 2008. The effect of topical application of pure honey on radiation-induced mucositis: a randomized clinical trial. J. Contemp. Dent. Pract. 9:40–47 [PubMed] [Google Scholar]
- 27. Peldyak J, Makinen KK. 2002. Xylitol for caries prevention. J. Dent. Hyg. 76:276–285 [PubMed] [Google Scholar]
- 28. Robson V, Dodd S, Thomas S. 2009. Standardized antibacterial honey (Medihoney) with standard therapy in wound care: randomized clinical trial. J. Adv. Nurs. 65:565–575 [DOI] [PubMed] [Google Scholar]
- 29. Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. 1991. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Methods 142:257–265 [DOI] [PubMed] [Google Scholar]
- 30. Scheinin A, Makinen KK, Ylitalo K. 1976. Turku sugar studies. V. Final report on the effect of sucrose, fructose and xylitol diets on the caries incidence in man. Acta Odontol. Scand. 34:179–216 [DOI] [PubMed] [Google Scholar]
- 31. Shannon IL, Edmonds EJ, Madsen KO. 1979. Honey: sugar content and cariogenicity. ASDC J. Dent. Child. 46:29–33 [PubMed] [Google Scholar]
- 32. Siddiqui IR. 1971. The sugars of honey. Adv. Carbohydr. Chem. Biochem. 25:285–309 [Google Scholar]
- 33. Steinberg D, Kaine G, Gedalia I. 1996. Antibacterial effect of propolis and honey on oral bacteria. Am. J. Dent. 9:236–239 [PubMed] [Google Scholar]
- 34. van Houte J. 1994. Role of micro-organisms in caries etiology. J. Dent. Res. 73:672–681 [DOI] [PubMed] [Google Scholar]
- 35. Wahdan HA. 1998. Causes of the antimicrobial activity of honey. Infection 26:26–31 [DOI] [PubMed] [Google Scholar]
- 36. White JW, Jr, Subers MH, Schepartz AI. 1963. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta 73:57–70 [DOI] [PubMed] [Google Scholar]
- 36a. White JW, Jr, Doner LW. 1980. Honey composition and properties, p 82–91 Beekeeping in the United States. US Department of Agriculture, Washington, DC [Google Scholar]
- 37. Wilkinson JM, Cavanagh HM. 2005. Antibacterial activity of 13 honeys against Escherichia coli and Pseudomonas aeruginosa. J. Med. Food 8:100–103 [DOI] [PubMed] [Google Scholar]
- 38. Willix DJ, Molan PC, Harfoot CG. 1992. A comparison of the sensitivity of wound-infecting species of bacteria to the antibacterial activity of manuka honey and other honey. J. Appl. Bacteriol. 73:388–394 [DOI] [PubMed] [Google Scholar]
- 39. Zumla A, Lulat A. 1989. Honey—a remedy rediscovered. J. R. Soc. Med. 82:384–385 [DOI] [PMC free article] [PubMed] [Google Scholar]