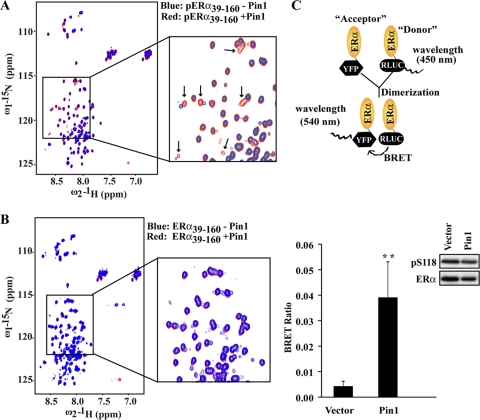
Fig 5.
Pin1 induces NMR spectral changes in the pERα fragment and induces hormone-independent dimerization of ERα. (A and B) Overlay of 2D 1H-15N HSQC spectra (600 MHz 1H), recorded at 25°C, of phosphorylated ERα39–160 (pERα39–160) alone (blue) or with excess Pin1 (red) (A) or unphosphorylated ERα39–160 (ERα39–160) alone (blue) or with excess Pin1 (red) (B). In panel A, the box encloses several cross peaks, indicated by arrows, that exhibited significant chemical shift changes upon Pin1 addition. (C) ERα dimerization was assessed in a BRET assay. (Upper panel) Cartoon representation of BRET assay results, with ERα molecules tagged with either “donor” Renilla luciferase (RLUC) or “acceptor” YFP. At close proximity, YFP emits light at the 540-nm wavelength. (Lower panel) BRET assays were carried out in HEK293 cells transiently expressing ER-RLUC and ERα-YFP that were transfected with vector or Flag-Pin1. The BRET ratio was calculated as described in Materials and Methods and includes correction for background random collision. The inset shows Western blot results for pS118-ERα and ERα. Data are presented as means ± SEM for at least three independent experiments. ∗∗, P < 0.01.