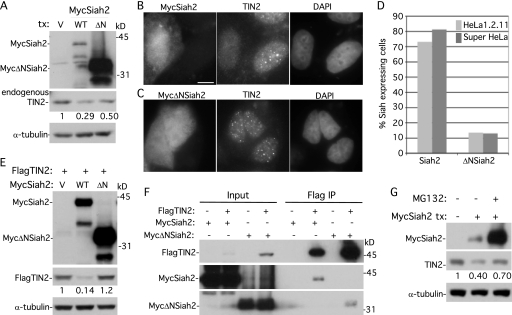
Fig 5.
Overexpression of Siah2 leads to loss of TIN2 at telomeres dependent upon the Siah2 RING domain and on the proteasome. (A) Immunoblot analysis of extracts from supertelomerase HeLa cells transfected with vector (V), MycSiah2 (WT), or MycΔNSiah2 (ΔN) and probed with anti-Myc, anti-TIN2 701, or anti-α-tubulin. (B and C) Immunofluorescence analysis of supertelomerase HeLa cells transfected with MycSiah2 (B) or MycΔNSiah2 (C). Cells were formaldehyde fixed and dually stained with anti-Myc and anti-TIN2 701 antibodies and with DAPI. Bar, 5 μm. (D) Graphical representation of the frequency of Siah2-expressing cells lacking TIN2 at telomeres (n = 126 cells or more each). (E) Immunoblot analysis of extracts from supertelomerase HeLa cells transfected with FlagTIN2 (+) and vector (V), MycSiah2 (WT), or MycΔNSiah2 (ΔN) and probed with anti-Myc, anti-Flag, or anti-α-tubulin. (F) Although MycΔNSiah2 does not affect TIN2 protein levels or localization to telomeres, it nonetheless binds to TIN2 by coimmunoprecipitation. 293T cells were transfected with FlagTIN2 and with MycSiah2 or MycΔNSiah2. Cell lysates were immunoprecipitated with anti-Flag beads and analyzed by immunoblotting with anti-Flag or anti-Myc antibody. (G) Inhibition of the proteasome by MG132 rescues Siah2-induced loss of TIN2. Supertelomerase HeLa cells were transfected for 16 h with vector (−) or MycSiah2 (+). MG132 (10 μm) was added 4 h prior to harvest. Cell extracts were analyzed by immunoblotting with anti-Myc, anti-TIN2 701, and anti-α-tubulin. (A, E, and G) TIN2 protein levels relative to α-tubulin and normalized to the vector control are indicated below the blots.