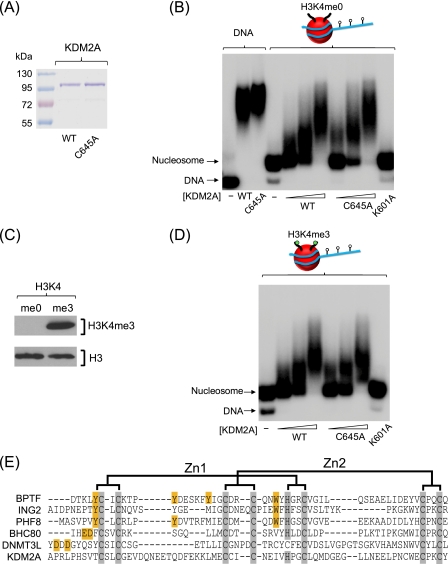
Fig 3.
The KDM2A PHD domain does not contribute significantly to nucleosome binding. (A) KDM2A proteins encoding a wild-type (WT) or mutant PHD domain (C645A) were expressed and purified. (B) Both the WT and C645A KDM2A proteins bind to naked 216-bp nucleosome positioning DNA with the same level of efficiency, as determined by EMSA (left-hand lanes). Mutating the PHD domain did not inhibit binding to the 216-bp mononucleosome, whereas mutation of the ZF-CXXC DNA biding domain (K601A) completely abrogated binding. (C) Histone H3K4me3 was installed specifically into histone H3 and the incorporation verified by Western blotting of the recombinant histone with antibodies against H3K4me3 or histone H3. (D) Mononucleosomes (216 bp) were reconstituted with histones containing H3K4me3 as indicated by the green dots on the nucleosome cartoon above the EMSA panels. The addition of H3K4me3 to the mononucleosome did not increase binding of KDM2A, nor did mutation of the PHD domain inhibit binding to the nucleosome, indicating that H3K4me3 does not contribute significantly to nucleosome recognition by KDM2A. (E) Multiple sequence alignment of PHD domains that bind either to H3K4me3 (BPTF, ING2, and PHF8) or H3K4me0 (BHC80 and DNMT3L) and KDM2A. Gray boxes indicate conserved zinc-coordinating cysteines/histidines, and orange boxes indicate methyl-lysine- or lysine-interacting residues. Although KDM2A has conserved zinc-coordinating residues, lysine interaction residues are absent.