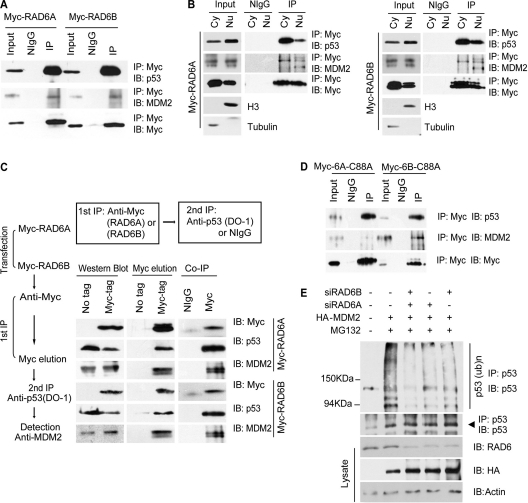
Fig 2.
RAD6 forms a ternary complex with MDM2 and p53. (A) RAD6 interacts with p53 and MDM2 in vivo. HeLa cells were transfected with Myc-RAD6 plasmids. The immunoblots were stained with anti-p53, anti-MDM2, or anti-Myc antibodies to visualize these proteins. The result showed that p53 (upper panel) and MDM2 (middle panel) could be precipitated by an anti-Myc antibody. The antibody against the Myc tag is indicated in the lower panel. (B) The interaction between RAD6, MDM2, and p53 occurs in both the cytoplasm and the nucleus. HeLa cells were transfected with Myc-RAD6A and Myc-RAD6B plasmids. Cytoplasmic (Cy) and nuclear (Nu) extracts were differentially prepared, and the coimmunoprecipitation experiment was performed with an anti-Myc antibody. The immunoblots were stained with anti-p53, anti-MDM2, or anti-Myc antibodies to visualize these proteins. The results showed that p53 and MDM2 could be precipitated by an anti-Myc antibody in both the cytoplasm and the nucleus. The antibody against the Myc tag is indicated. (C) RAD6 forms a ternary complex with MDM2 and p53. A two-step coimmunoprecipitation experiment was employed to test for ternary complex formation. The procedures of the two-step coimmunoprecipitation are outlined on the left. HeLa cells were transfected with or without (no tag) Myc-RAD6. The first immunoprecipitation was performed with an anti-Myc antibody. The complex was eluted using the Myc peptide. The second step of immunoprecipitation used an anti-p53 antibody or the control mouse IgG (NIgG) to precipitate the complex. Protein samples from each step were then separately subjected to Western blot analysis using anti-Myc, anti-p53, and anti-MDM2 antibodies. The experiment was repeated with essentially the same results. (D) RAD6 ubiquitin-conjugating enzymatic activity is not required for the interaction with MDM2 and p53. HeLa cells were transfected with Myc-RAD6 mutants, Myc-RAD6-C88A (Myc-6A-C88A or Myc-6B-C88A), and coimmunoprecipitation experiments were employed using an anti-Myc antibody. The anti-p53 (upper panel) and anti-MDM2 (middle panel) antibodies were used to visualize these proteins in the Western blot analysis. The antibody against the Myc tag is indicated in the lower panel. (E) MDM2-induced p53 ubiquitination is dependent on RAD6. HL-7702 cells were transfected with (+) or without (−) HA-MDM2 in the presence (+) or absence (−) of RAD6 siRNAs and treated with DMSO (−) or MG132. The anti-p53 antibody was used to visualize the amounts of precipitated p53 and ubiquitinated p53. The expressions of p53 and HA-MDM2 (HA) are shown in the lower panels.