Fig 7.
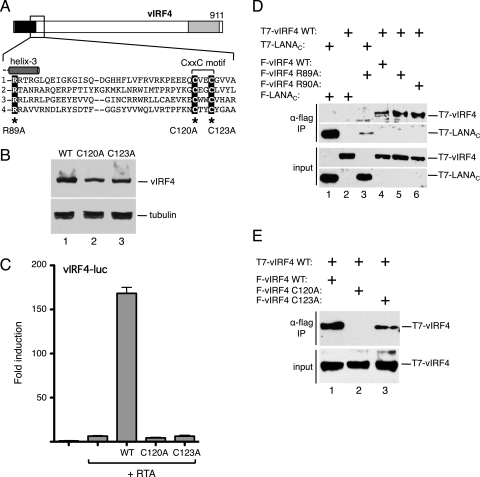
The signature cysteine motif in vIRF4 is essential for the transactivation function. (A) Schematic of vIRF4. The inset shows the sequence immediately downstream of helix 3, including the CxxC motif common to all KSHV vIRFs (and RRV vIRFs [data not shown]). Corresponding sequences of KSHV vIRF1, vIRF2, and vIRF3 are shown using the invariant arginine (R89 in vIRF4) to anchor the alignment. Cysteine-120 and cysteine-123 were individually changed to alanine. (B) Immunoblot (with anti-Flag antibody) of lysates prepared from cells transfected with each vIRF4 plasmid. The species corresponding to full-length vIRF4 is indicated. Equal loading was demonstrated by blotting for α-tubulin. (C) Activity assay using HeLa cells transfected with vIRF4-luc (250 ng) and each vIRF4 derivative (200 ng) in the presence of RTA (25 ng). Fold induction was calculated relative to the reporter alone. Values represent the means and standard errors of the means of three independent transfections. (D) vIRF4 is capable of self-association. Lysates were prepared from HeLa cells transfected with plasmids encoding combinations of T7 and Flag-tagged proteins and subjected to immunoprecipitation using Flag-coupled beads. Precipitates were resolved by SDS-PAGE and blotted with anti-T7 antibody. Expression was confirmed by blotting lysates directly. (E) Self-association is disrupted by mutation of the signature cysteine residues. Coimmunoprecipitation analysis was conducted as described for panel D, using wild-type (WT) and mutant (C120A and C123A) versions of vIRF4.