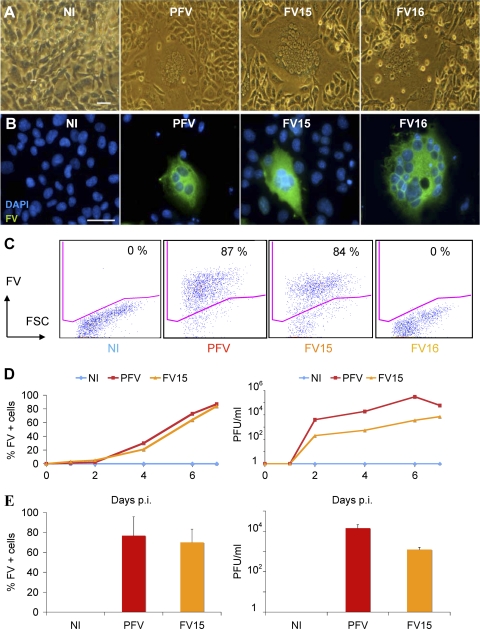
Fig 1.
Description of FV strains used in the present study. (A and B) Visualization of FV-infected cells. BHK21 cells were noninfected (NI) or infected with prototypic PFV, FV15, or FV16 primary strains. After 3 to 5 days of infection, large syncytia were visible. (A) Light microscopy analysis. (B) Immunofluorescence analysis. FV-infected cells were stained with an anti-FV polyclonal serum (green). Nuclei were stained with DAPI. Representative fields are shown. Scale bar, 5 μm. (C) Flow cytometry analysis of infected BHK21cells. After infection, cells were stained with an anti-FV polyclonal serum, which recognizes PFV- and FV15-infected cells, but not cells infected with the genetically distant FV16 strain. The percentage of FV+ cells is indicated. Staining was performed at day 3 postinfection for PFV and day 5 postinfection for FV15 and FV16. (D) Propagation of PFV and FV15 on BHK21cells. BHK21 cells were noninfected (NI) or infected with PFV (multiplicity of infection [MOI] = 0.05) or FV15 (MOI = 0.5) and then analyzed by flow cytometry at the indicated days postinfection (p.i.) (left panel). Cells were lysed by freeze-thaw cycles to harvest viruses. Viral infectivity (in PFU/ml) was determined using FAB-reporter cells (right panel). The results of a representative experiment are shown. The FV16 strain does not activate the PFV promoter present in FAB cells (data not shown). (E) Analysis of BHK21 cells chronically infected with PFV or FV15. BHK-infected cell cultures were maintained by addition of noninfected cells twice a week. Cells were analyzed by flow cytometry (left panel) and lysates were titrated on FAB-reporter cells (right panel). Means ± the standard deviations (SD) of three independent experiments are shown.