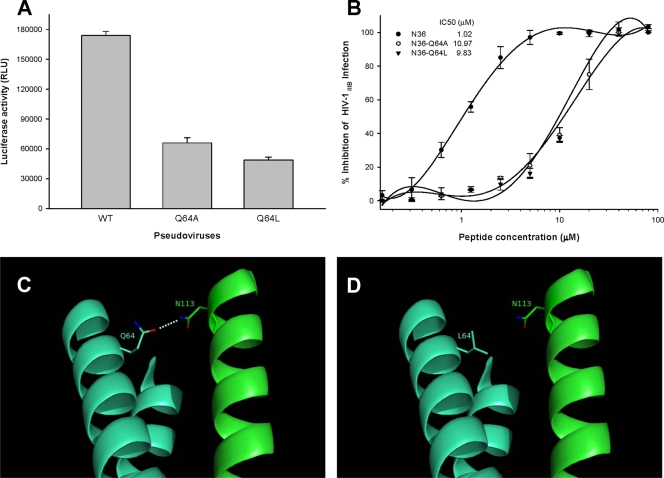
Fig 2.
Effect of Q64 mutations on viral infectivity, N-peptide-mediated anti-HIV-1 activity, and interactions between NHR and CHR. (A) Infectivity of HIV-1 pseudoviruses bearing a wild-type (WT) or mutant sequence of gp41. Single-cycle infection of the viruses in MT-2 cells was measured by a luciferase-based assay. (B) Anti-HIV-1 activity of wild-type and mutant N36 peptides. The infectivity of HIV-1IIIB in MT-2 cells in the presence or absence of the peptides was measured by a p24-based assay. The IC50 of each peptide is shown in the figure. The experiment was performed in triplicate, and the data are presented as means ± standard deviations (SD). (C and D) Predicted interactions between N113 in the CHR domain and Q64 (C) or L64 (D) in the NHR domain. Molecular modeling analysis of the complex formed by CP32 (amino acids 110 to 141) and T21 (14) was performed using PyMol software (38). Two NHR domains are shown in cyan blue, while the CHR peptide is shown in green. A hydrophilic interaction between Q64 in NHR and N113 in CHR is indicated. Mutation of Q64 with the hydrophobic residue leucine resulted in the loss of the polar contact.