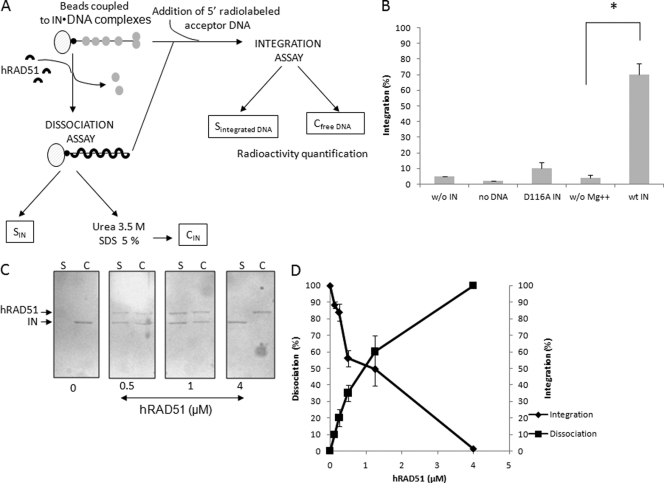
Fig 4.
Dissociation effect of hRAD51 on active IN-DNA integration complex. The 296-bp viral DNA used in the concerted integration assay and fused to biotin was coupled with magnetic beads. HIV-1 IN was then bound to the DNA (checked by SDS-PAGE). hRAD51 treatment was performed under different conditions promoting the formation of the active nucleofilament or not. IN-DNA dissociation and hRAD51 binding were checked by SDS-PAGE of the supernatant (C, lanes S). The proteins remaining bound to the DNA were analyzed after SDS and urea treatment of the bead pellet and SDS-PAGE followed by Western blotting (C, lanes C). The presence of IN and hRAD51 in the bead pellet and the supernatant was checked after SDS-PAGE of the corresponding fractions and Western blotting using a mixture of antibodies against each protein (strategy is shown in panel A). Coupling of active complexes was checked by integration assays using different conditions promoting (wt IN) or not (w/o Mg++; use of inactivated D116A enzyme) IN activity in the presence of a 5′-radiolabeled acceptor DNA. The activity of the coupled IN was measured by quantifying the radioactivity remaining covalently bound to the beads after repeated washings (B). A Student test was performed on serial values. *, P < 0.005. Treatment of the complexes with increasing concentrations of hRAD51 in the presence of 100 μM ATP led to the dissociation of IN from its substrate (C). (D) Quantification of the integration activity catalyzed by the IN coupled to the beads and IN dissociation after treatment with increasing concentrations of hRAD51.