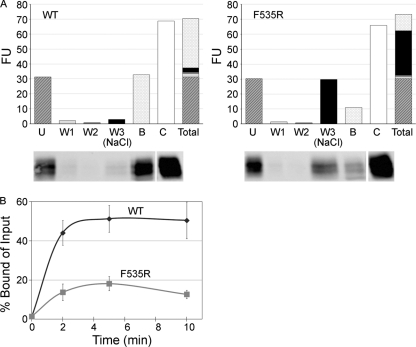
Fig 5.
The 19-kDa GP Ecto of a fusion loop mutant (F535R) is impaired in liposome binding. (A) Equivalent amounts (0.8 μg) of WT and F535R 19-kDa GP Ecto proteins were incubated with liposomes at pH 5.0 and 50°C for 10 min. Mixtures were then incubated with streptavidin magnetic beads as described in Materials and Methods and the following fractions analyzed by SDS-PAGE and Western blotting for GP1: unbound (U), 1st wash with HM buffer (W1), 2nd wash with HM buffer (W2), 3rd wash with HM buffer containing 1 M NaCl [W3 (NaCl)], and remaining bound protein (B). The fluorescence units (FU) of corresponding gel samples (below the graphs) are shown. The control (C) was the supernatant from a sample maintained for 10 min at 4°C and pH 7.5. “Total” shows a stacked column of the fluorescent units found in the U, W1, W2, W3, and B fractions (i.e., the sum equals all of the GP1 recovered). The control gel sample was repositioned from the far left on the gel to the far right of the image (indicated with a space) to align with the corresponding bars in the overlying graph. (B) Equivalent amounts of WT and mutant F535R 19-kDa GPΔ Ecto proteins were incubated with liposomes for the indicated times at 50°C and pH 5.0. The protein-liposome complexes were then collected on magnetic beads, which were washed twice with HM buffer and once with HM buffer containing 1 M NaCl. Samples were then separated on SDS gels and blotted for GP1 as described above. The results were calculated as [(bound GP)/(total input GP)] × 100, where total input GP is the unbound signal from parallel samples maintained at pH 7.5 and 4°C and processed in the identical manner. Data are the averages from three experiments, each performed in duplicate. Error bars indicate SD.