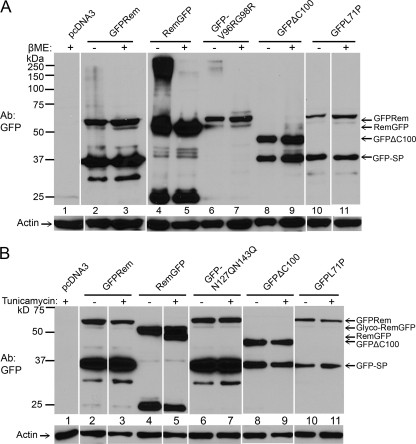
Fig 7.
GFP tagging at the Rem C terminus leads to the accumulation of glycosylated Rem precursor in a disulfide-bonded protein complex. (A) C-terminal GFP tagging allows accumulation of a disulfide-bonded protein complex with Rem. The 293 cells were transfected with 2.5 μg of GFP-tagged rem constructs as indicated. Cells were transfected with relatively large amounts of expression plasmids to allow detection of mutant proteins by Western blotting. After 48 h, cells were lysed in SDS-containing loading buffer in the presence or absence of β-mercaptoethanol (βME). Extracts (30 μg) were used for Western blotting with GFP-specific (upper panel) or actin-specific (lower panel) antibody. The positions of specific bands are indicated by arrows. (B) C-terminally GFP-tagged Rem is glycosylated. The 293 cells were transfected with 1 μg of the indicated rem expression constructs. After 24 h, half of the transfected cells were treated with tunicamycin (5 μg/ml). After an additional 24-h incubation, cells were lysed in SDS-containing loading buffer containing βME and used for Western blotting as described for panel A. The partial change in RemGFP mobility presumably resulted from protein synthesis prior to addition of tunicamycin or a partially effective drug concentration.