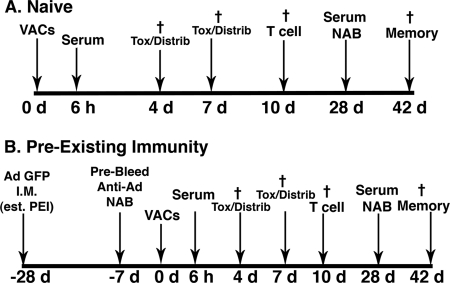
Fig 1.
Timeline for administration of virus-antibody complexes (VACs), sample collection, necropsy, and assessment of the immune response for naïve mice (A) and those with preexisting immunity (PEI) (B). Mice were given a single dose of 1 × 1011 virus particles mixed with different concentrations of anti-adenovirus antibodies (naïve mice) or 28 days after establishment of preexisting immunity by tail vein injection in a volume of 100 μl. Control mice were given 100 μl of sterile PBS and gentamicin in the same manner. Six hours after treatment, blood was collected from the saphenous vein and serum processed for assessment of cytokines, platelets, and serum transaminases. Blood was also collected on days 3 and 7 for assessment of serum transaminases and platelets. Mice from each group (n = 4) were sacrificed on days 4 and 7, and portions of liver and spleen were cryopreserved for sectioning and histochemical detection of beta-galactosidase. Remaining tissue and other organs (heart, kidney, lung, lymph nodes) were harvested and snap-frozen for assessment of virus distribution by real-time PCR. Ten days after treatment, mice (n = 5) were sacrificed and splenocytes processed for assessment of T cell responses. Tissue samples were also taken at this time point for assessment of virus distribution patterns. On day 28, blood was collected from the saphenous vein of remaining animals for characterization of anti-beta-galactosidase and anti-adenovirus antibodies. These animals (5 per group) were sacrificed on day 42 and splenocytes processed for assessment of immunological memory by CFSE staining and flow cytometry. †, terminal bleed and necropsy of animals at the denoted time point.