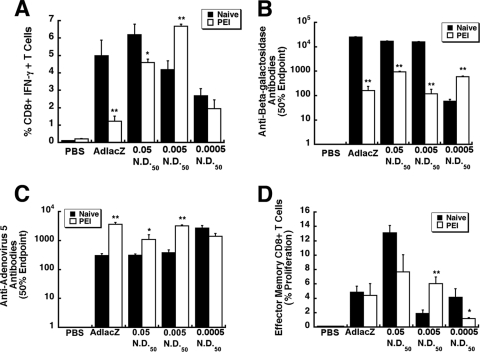
Fig 7.
Preexisting immunity to adenovirus serotype 5 does not significantly compromise the immune response elicited by some VACs. Preexisting immunity was established by intramuscular injection of 1 × 1011 particles of AdEGFP 28 days prior to administration of VACs. At that time, mice had a circulating anti-adenovirus NAB titer of a 184.2 ± 32.4 reciprocal dilution. Animals given saline served as negative controls (PBS). (A) Frequency of IFN-γ-secreting CD8+ T cells. Ten days after treatment, 1 × 106 splenocytes from each animal were harvested and incubated with a beta-galactosidase-specific peptide. Responsive cells were quantitated by flow cytometry. (B) Anti-beta-galactosidase antibody profile after administration of VACs. Serum was analyzed 28 days after treatment. Fifty percent endpoint titers are plotted according to treatment and were calculated according to the method of Reed and Muench. (C) Anti-adenovirus neutralizing antibody. Neutralizing antibody titers were determined by assessing the ability of sera to block infection of HeLa cells by unmodified virus expressing beta-galactosidase. The 50% endpoint titer is plotted according to treatment. (D) Memory response. Cells positive for CD8+, CD44hi, and CD62Llo were evaluated for CFSE staining by flow cytometry. Data represent the degree of effector CD8+ T cell expansion after stimulation for each treatment group. Data illustrated in each panel reflect the means and standard errors of the means for five animals/group. Statistical significance was determined between individual treatment groups and vehicle controls or between naïve animals and those with preexisting immunity to adenovirus by one-way analysis of variance with a Bonferroni/Dunn post hoc test. *, P ≤ 0.05; **, P ≤ 0.01. PEI, preexisting immunity to adenovirus.