Abstract
This study presents an evaluation of the MIC1 (microneme protein 1)-MAG1 (matrix antigen 1) Toxoplasma gondii recombinant chimeric antigen for the serodiagnosis of human toxoplasmosis for the first time. The recombinant MIC1-MAG1 antigen was obtained as a fusion protein containing His tags at the N- and C-terminal ends using an Escherichia coli expression system. After purification by metal affinity chromatography, the chimeric protein was tested for usefulness in an enzyme-linked immunosorbent assay (ELISA) for the detection of anti-T. gondii immunoglobulin G (IgG). One hundred ten sera from patients at different stages of infection and 40 sera from seronegative patients were examined. The results obtained for the MIC1-MAG1 chimeric antigen were compared with those of IgG ELISAs using a Toxoplasma lysate antigen (TLA), a combination of recombinant antigens (rMIC1ex2-rMAG1) and single recombinant proteins (rMIC1ex2 and rMAG1). The sensitivity of the IgG ELISA calculated from all of the positive serum samples was similar for the MIC1-MAG1 chimeric antigen (90.8%) and the TLA (91.8%), whereas the sensitivities of the other antigenic samples used were definitely lower, at 69.1% for the mixture of antigens, 75.5% for the rMIC1ex2, and 60% for rMAG1. This study demonstrates that the MIC1-MAG1 recombinant chimeric antigen can be used instead of the TLA in the serodiagnosis of human toxoplasmosis.
INTRODUCTION
Toxoplasmosis, which is caused by Toxoplasma gondii, an obligate intercellular protozoan parasite, is generally asymptomatic in healthy individuals but may cause severe complications in immunocompromised individuals such as AIDS patients and transplant recipients. A serious problem is also posed by congenital toxoplasmosis as a result of transplacental fetus infection in pregnant women. Congenital disease may cause spontaneous abortion and serious damage to the fetus, with the occurrence of severe neurological disorders. Therefore, an accurate diagnosis of recently acquired infection during pregnancy is crucial, as well as being very important for the clinical management of the mother and her fetus. Toxoplasmosis is generally diagnosed by the demonstration of specific antibodies to Toxoplasma antigens in the serum samples of infected patients. The presence of a recent infection can be determined by detecting the seroconversion of immunoglobulin M (IgM) or IgG antibodies, a substantial increase in IgG antibody titer, or a Toxoplasma serologic profile compatible with the acute phase of infection, using Toxoplasma serodiagnostic tests, including an IgG avidity test, in sequential serum samples of infected individuals (23, 28). However, this procedure has limitations with respect to estimation of the time of T. gondii infection, owing to the fact that, in many cases, low IgM titers persist long beyond the acute phase of disease (21). The confirmation of IgM antibodies in a human serum sample is thus an inadequate criterion for the diagnosis of acute toxoplasmosis.
The outcome of serological tests depends on the types of antigen and methods of detection of specific Igs. Most commercial tests use the Toxoplasma lysate antigen (TLA) obtained from a single stage of the life cycle, the tachyzoite. Although the TLA is characterized by high sensitivity and specificity in an enzyme-linked immunosorbent assay (ELISA), its disadvantages are high cost and lengthy production time, as well as the need to provide a parasite culture. Therefore, as soon as DNA technology became available for the production of new diagnostic tools, which is to say the recombinant proteins of T. gondii, they were considered as having the potential to replace the native antigens obtained from lysed whole parasites. The use of a recombinant antigen(s) for the diagnosis of T. gondii infections would prove highly beneficial to improving the standardization of the method, since the antigen composition of the test is precisely known. Furthermore, the advantages of these proteins are that (i) the production cost of antigens can be reduced and (ii) more than one defined antigen can be used for the detection of specific antibodies.
Chimeric antigens are a new kind of diagnostic tool. These proteins are a new generation of recombinant products which have the potential to replace the native antigen(s) received from lysed whole parasites. A single recombinant chimera contains different immunoreactive epitopes from various antigens of T. gondii. The combination of epitopes from proteins of different stages of the T. gondii life cycle is an optimal strategy for overcoming the antigen complexity of the parasite. Thus, the chimeric protein may be a more immunodominant antigen than the original antigens. Furthermore, the major advantage of using a chimeric antigen for antibody detection, rather than the existing commercial assays and tests based on a combination of recombinant products, would be a more “standardized” antigen. Heretofore, only one study has found that two chimeric antigens, namely, EC2 and EC3, which contain six antigenic regions of the T. gondii MIC2, MIC3, M2AP, dense granule antigen 3 (GRA3), GRA7, and surface antigen 1 (SAG1) proteins, improve the serological diagnosis of toxoplasmosis in adults with an acquired infection and infants born to mothers with a primary T. gondii infection contracted during pregnancy (3). Moreover, owing to the complexity of the parasite life cycle and the variability of the parasite antigens, multiepitope products have become an attractive strategy in the development of vaccines against toxoplasmosis (6, 31).
This study evaluated the usefulness of the new T. gondii recombinant chimeric antigen MIC1 (microneme protein 1)-MAG1 (matrix antigen 1) in an IgG ELISA as a means of improving the diagnosis of toxoplasmosis in humans.
MATERIALS AND METHODS
Construction of the recombinant plasmid.
The nucleotide sequence of the T. gondii gene encoding a fragment of the MAG1 antigen was obtained from the GenBank database (accession number AF251813). Tachyzoites from the T. gondii RH strain were used to isolate a genomic DNA which was used as the template for the amplification of the mag1 fragment gene using a standard PCR amplification protocol with the Hypernowa DNA polymerase (BLIRT SA, Gdansk, Poland). The PCR product was inserted into the pUET1/MIC1ex2 recombinant plasmid (11).
A DNA fragment of mag1 corresponding to nucleotides 590 to 599 and 710 to 1277 and encoding part of the tissue cyst matrix protein was obtained by means of PCR using primers 5′-GAA GTG ATA TCG AGC CAA AGG GTG CCA GAG CTA C-3′ (forward) and 5′-CAC CCC AAG CTT ACC AGA TCC CTG AAC CCT TAG-3′ (reverse). The primers contained EcoRV and HindIII recognition sequences (underlined) to facilitate cloning. The PCR product was digested with both EcoRV and HindIII and inserted into the EcoRV and HindIII sites of the pUET1/MIC1ex2 recombinant plasmid (11). The resulting plasmid, pUET1/MIC1-MAG1, contained a sequence of the N-terminal part of MIC1, amino acid residues 25 to 182, and a sequence of MAG1, amino acid residues 30 to 222, which was embedded in frame between the His tag domains for purification of the recombinant protein by means of metal affinity chromatography.
Expression and purification of the chimeric antigen and recombinant proteins.
E. coli strain Rosetta(DE3)pLacI, transformed with pUET1/MIC1-MAG1 or pUET1, was grown in LB supplemented with ampicillin (100 μg/ml) and chloramphenicol (34 μg/ml) overnight at 37°C. Next, 20 ml of the overnight culture was diluted by adding 1,000 ml of LB containing the same antibiotics. It was then incubated, with vigorous shaking, at 37°C. When the optical density at 600 nm (OD600) reached 0.4, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the culture to a final concentration of 1 mM and the cells were incubated, with vigorous shaking, for 16 h at the same temperature. The cells were then harvested by centrifugation, and the pellets were resuspended in 30 ml of buffer A (20 mM Tris-HCl [pH 7.9], 500 mM NaCl, 5 mM imidazole, 0.1% Triton X-100). After centrifugation, the protein was purified from the supernatant with the use of a Ni2+-iminodiacetic acid-Sepharose column in accordance with the manufacturer's instructions (Novagen).
Two other recombinant proteins (rMAG1 and rMIC1ex2) were obtained as previously described (11, 12). A TLA was prepared from tachyzoites (RH strain) as described earlier (15).
The recombinant proteins were analyzed by means of SDS-PAGE on 12% acrylamide gels and stained with Coomassie blue. The concentrations of the purified proteins were determined by the Bradford method using bovine serum albumin as the standard.
Western blot analysis.
For Western blot analysis, separated proteins were electrotransferred to a nitrocellulose membrane (30 min, 0.8 mA/cm gel2). After blocking with 5% fat-free milk in phosphate-buffered saline (PBS)–0.05% Tween, the membrane was incubated for 1 h at room temperature with serum samples diluted 1:100 in blocking solution, washed, and incubated with peroxidase-conjugated goat anti-human IgG secondary antibodies (Jackson ImmunoResearch) used at 1:2,000. A peroxidase activity was detected with H2O2 and 3,3′-diaminobenzidine tetrahydrochloride as a chromogenic substrate (Sigma).
Human serum samples.
All of the sera used in this study were received during routine toxoplasmosis screening. A total of 150 serum samples were analyzed and divided into four groups in accordance with the results obtained using the commercial tests. Group I serum samples were collected from 26 patients in the acute phase of toxoplasmosis. The presence of specific IgM antibodies was measured using VIDAS TOXO IgM (bioMérieux, Marcy l'Etoile, France). All of these sera had a specific IgG (VIDAS TOXO IgG II; bioMérieux) with low avidity (VIDAS TOXO IgG AVIDITY; bioMérieux). Group II consisted of 17 serum samples received from patients in the postacute phase of toxoplasmosis. The selection was based on the presence of specific IgG antibodies (VIDAS TOXO IgG II; bioMérieux) with low or borderline avidity (VIDAS TOXO IgG AVIDITY) and an absence of specific IgM antibodies (VIDAS TOXO IgM; bioMérieux). Group III included 67 sera from patients who had acquired toxoplasmosis in the distant past. All of these serum samples had IgG antibodies (VIDAS TOXO IgG II; bioMérieux) with high avidity (VIDAS TOXO IgG Avidity; bioMérieux). Specific IgM antibodies were not detected (VIDAS TOXO IgM; bioMérieux). Moreover, the serum samples from this group were further divided into three subgroups; IIIA consisted of 15 serum samples with high titers of IgG antibodies (>300 IU/ml), IIIB included 32 serum samples with a titer of IgG antibodies of between 51 to 300 IU/ml, and IIIC consisted of 20 serum samples with low titers of IgG antibodies (≤50 IU/ml). The last group, IV, was a control group of 40 human serum samples from patients who were not infected with T. gondii.
IgG ELISA.
MaxiSorp multiwell plates (Nunc, Roskilde, Denmark) were coated with rMAG1, rMIC1ex2, chimeric antigen MIC1-MAG1, and a mixture (M) of proteins (rMAG1 and rMIC1ex2) or with a TLA at final concentrations of 2.5 μg/ml for each recombinant protein and 1 μg/ml for the TLA in a coating buffer (0.05 M carbonate buffer, pH 9.6). Control plates were coated with a protein sample of E. coli Rosetta(DE3)pLacI transformed with a pUET1 vector obtained using the same purification method and dilution as for the recombinant antigens. After overnight incubation at 4°C, the plates were washed three times with PBS–0.1% Triton X-100 and blocked for 1 h at 37°C in blocking solution (1% bovine serum albumin, 0.5% Triton X-100 in PBS). The cells were then washed three times and incubated for 1 h at 37°C with the human serum diluted 1:100 in blocking solution. Next, the plates were washed three times with washing buffer and incubated with anti-human IgG peroxidase-labeled conjugates (Jackson ImmunoResearch) diluted 1:16,000 in blocking solution for 1 h at 37°C, after which o-phenylenediamine dihydrochloride chromogenic substrate (Sigma) was added. After 45 min of incubation at 37°C in darkness, the reaction was stopped by the addition of 0.1 M sulfuric acid and the OD492 was measured using a microtiter plate reader (Multiskan FC; Thermo Scientific).
Each serum sample was examined twice. The results were determined for each sample by calculating the mean OD reading of duplicate wells. A positive result was defined as any value higher than the average OD reading plus 2 standard deviations (cutoff) obtained with 20 serum samples from the control, group IV, which consisted of seronegative serum samples. The calculated cutoff values were 0.545 for rMIC1ex2, 0.558 for rMAG1, 0.454 for MIC1-MAG1, 0.579 for the rMIC1ex2-rMAG1 mixture, and 0.546 for the TLA. The ELISA carried out with control E. coli Rosetta(DE3)pLacI/pUET1 antigen revealed an OD of <0.425. This means that there was no influence of possible contamination of the recombinant antigens with E. coli proteins.
IgG avidity ELISA.
Fourteen serum samples from patients in the acute phase of toxoplasmosis (IgM positive and IgG with low avidity) and 14 serum samples from patients with chronic infections (IgM negative and IgG with high avidity) were examined in an IgG avidity ELISA. Each serum sample was used at increasing serial dilutions (1:50 to 1:800) in separate plates coated with MIC1-MAG1. An IgG avidity ELISA was performed using two procedures. One of them was a normal ELISA, as described above, and the other included an additional step, namely, washing three times with a urea solution (PBS, 0.1% Triton X-100, 6 or 8 M urea) for 10 min.
The results are shown as avidity indexes (AIs), which were calculated as the ratio of the OD of the serum sample washed with urea solution and the OD of the serum sample without additional washing (for the dilution which gave an OD of almost 1.0). In line with the results obtained earlier (13) and applied by Pietkiewicz et al. (27), <0.3 was considered low avidity, 0.3 to 0.4 was considered borderline avidity, and >0.4 was considered high avidity.
RESULTS
Expression and purification of the MIC1-MAG1 chimeric antigen.
A fragment of the gene encoding the MAG1 antigen was amplified by PCR and cloned into recombinant plasmid pUET1/MIC1ex2. As a result of its expression, a fusion of the MIC1-MAG1 chimeric antigen containing six histidyl residues at both ends (at the N and C termini) was produced as a soluble protein with a calculated molecular mass of 41.8 kDa. The recombinant chimeric antigen was purified by means of a one-step chromatography procedure using metal affinity chromatography with Ni2+ bound to iminodiacetic acid-agarose (Novagen). The yield of purified MIC1-MAG1 was 43 mg/liter of induced bacterial culture, with a purity of >95% (data not shown).
Immunoreactivities of T. gondii IgG antibodies in Western blot assays with the chimeric antigen and recombinant proteins.
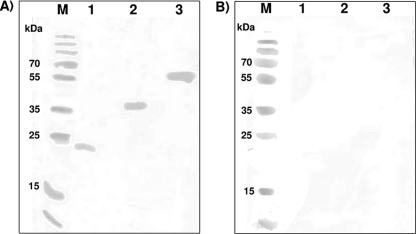
The reactivity of the purified MIC1-MAG1 chimeric antigen was tested using Western blotting with several human serum samples chosen from each of the four groups included in this study. As a positive control for chimeric antigen reactivity, two recombinant proteins (rMIC1ex2 and rMAG1) were used. IgG antibodies in the sera from patients in the acute, postacute, and chronic phases of toxoplasmosis, namely, groups I, II, and III, respectively, reacted strongly with chimeric antigen MIC1-MAG1 and the recombinant proteins (Fig. 1A). Furthermore, no immunoreactivity of any of the recombinant antigens with the IgG antibodies in the serum samples from the healthy patients in group IV was observed (Fig. 1B).
Fig 1.
Representative results of Western blotting analyses. Lanes M, molecular mass markers (Fermentas, Vilnius, Lithuania). Purified rMIC1ex2 (lane 1), rMAG1 (lane 2), and chimeric antigen MIC1-MAG1 (lane 3) were tested with serum samples from a T. gondii-infected individual (A) and a healthy patient (B).
Immunoreactivities of T. gondii IgG antibodies in ELISAs with the chimeric antigen, recombinant proteins, and TLA.
All of the groups of human serum samples, namely, groups I (26 sera from patients in the acute phase of toxoplasmosis), II (17 sera from patients in the postacute phase of toxoplasmosis), III (67 sera from patients in the chronic phase of toxoplasmosis), and IV (40 sera from seronegative individuals), were examined by means of an IgG ELISA conducted with the MIC1-MAG1 chimeric antigen, a mixture (M) of recombinant antigens (rMAG1 and rMIC1ex2), the separate antigens (rMAG1 and rMIC1ex2), and the TLA. A pool of 20 sera from group IV, which contained serum samples from healthy patients, was tested in order to calculate cutoff values for all of the IgG ELISAs, which were set as the mean value of the negative serum samples plus 2 standard deviations. Moreover, the remaining 20 sera from this group were used to determine the specificity of the IgG ELISAs. None of these sera reacted above the cutoff value, resulting in a specificity of 100% in the ELISAs (Table 1).
Table 1.
Comparison of the immunoreactivities of rMAG1, rMIC1ex2, the MIC1-MAG1chimeric antigen, a mixture of antigens rMIC1ex2 and rMAG1, and the TLA using sera from individuals in the acute, postacute, and chronic phases of toxoplasmosis and sera from healthy patients
| Serum sample groupa and antigen | No. (%) of reactive serum samples | Mean absorbance value (range)b |
|---|---|---|
| Ic | ||
| rMAG1 | 22 (84.6) | 1.259 (0.317–2.701) |
| rMIC1ex2 | 23 (88.5) | 1.236 (0.312–2.188) |
| M | 23 (84.6) | 1.434 (0.335–2.702) |
| MIC1-MAG1 | 26 (100) | 1.651 (0.503–2.911) |
| TLA | 23 (88.5) | 1.006 (0.304–1.627) |
| IId | ||
| rMAG1 | 6 (35.3) | 0.500 (0.340–0.890) |
| rMIC1ex2 | 14 (82.5) | 0.668 (0.311–1.329) |
| M | 9 (52.9) | 0.624 (0.414–1.236) |
| MIC1-MAG1 | 17 (100) | 0.738 (0.494–1.510) |
| TLA | 15 (88.2) | 0.796 (0.406–2.083) |
| IIIe | ||
| rMAG1 | 38 (56.7) | 0.732 (0.212–2.616) |
| rMIC1ex2 | 46 (68.7) | 0.781 (0.356–1.875) |
| M | 44 (65.7) | 0.916 (0.282–2.994) |
| MIC1-MAG1 | 57 (85.1) | 1.007 (0.299–2.693) |
| TLA | 63 (94.0) | 1.310 (0.377–2.419) |
| Totalf | ||
| rMAG1 | 66 (60.0) | 0.821 (0.212–2.702) |
| rMIC1ex2 | 83 (75.5) | 0.871 (0.311–2.188) |
| M | 76 (69.1) | 0.993 (0.282–3.155) |
| MIC1-MAG1 | 100 (90.9) | 1.118 (0.299–2.911) |
| TLA | 101 (91.8) | 1.159 (0.304–2.419) |
| IVg | ||
| rMAG1 | 0 | 0.312 (0.230–0.443) |
| rMIC1ex2 | 0 | 0.383 (0.176–0.481) |
| M | 0 | 0.358 (0.193–0.565) |
| MIC1-MAG1 | 0 | 0.300 (0.195–0.430) |
| TLA | 0 | 0.326 (0.141–0.429) |
n, number of serum samples examined.
The cutoff values were 0.558 for rMAG1, 0.545 for rMIC1ex2, 0.579 for M, 0.454 for MIC1-MAG1, and 0.546 for TLA.
Acute phase of toxoplasmosis; n = 26.
Postacute phase of toxoplasmosis; n = 17.
Chronic phase of toxoplasmosis; n = 67.
n = 110.
Controls negative for anti-T. gondii antibodies; n = 10.
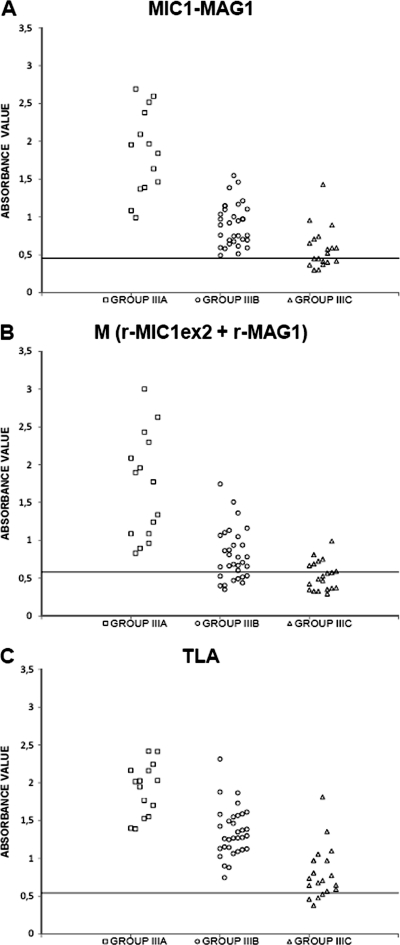
The sensitivity of the IgG ELISA with the MIC1-MAG1 chimeric antigen calculated for all of the positive serum samples, namely, groups I to III, was 90.9% (Table 1). This value was almost as high as that obtained for the IgG ELISA carried out with the TLA (91.8%). Moreover, the sensitivity of the IgG ELISAs applied to a single recombinant protein (rMAG1 or rMIC1ex2) or a mixture of these antigens (M) was much lower, at 60%, 75.5%, and 69.1%, respectively. Examination of the results obtained for sera from patients in the acute or postacute phase of toxoplasmosis, namely, groups I and II, respectively, showed that the IgG antibodies from all of the serum samples included in these groups reacted with the MIC1-MAG1 chimeric antigen (100%), whereas the reactivity of the specific IgG with the TLA was lower, at 88.5% for group I and 88.2% for group II. A similar sensitivity of >80% for the IgG ELISAs carried out with the separate recombinant proteins (rMAG1 and rMIC1ex2) and with a mixture of these antigens (M) was obtained for the sera from group I, whereas it differed for the sera from group II, at 35.3%, 82.5%, and 52.9% for rMAG1, rMIC1ex2, and M, respectively. Furthermore, the sera from the patients in the chronic phase of toxoplasmosis, namely, group III, reacted with the MIC1-MAG1 chimeric antigen and the TLA with high sensitivities of 85.1% and 94%, respectively, whereas much lower sensitivities were observed for the single recombinant proteins (rMAG1 and rMIC1ex2) and the mixture, at 56.7%, 68.7%, and 65.7%, respectively. However, the results obtained with different antigenic samples and this group of serum samples were dependent on the IgG antibodies titers. Thus, all (100%) of the sera with titers of >300 IU/ml reacted with the chimeric antigen, the mixture of antigens, and the TLA (Fig. 2). The same sensitivity was obtained for the MIC1-MAG1 antigen and the TLA when the serum samples with medium titers of 51 to 300 IU/ml were tested, whereas it was lower for the mixture of recombinant proteins, at 87.5%. Furthermore, the lowest sensitivities were obtained with the serum samples of subgroup IIIC, with a titer of <50 IU/ml, at 80% for the TLA, 50% for the chimeric antigen, and only 30% for the mixture of recombinant proteins.
Fig 2.
Immunoreactivities of the MIC1-MAG1 chimeric protein (A), the mixture of antigens (B), and the TLA (C) with sera from the patient group in the chronic phase of toxoplasmosis, divided according to IgG titers as follows: □, >300 IU/ml; ○, 51 to 300 IU/ml; ▵, ≤50 IU/ml. Absorbance was measured at 492 nm. The horizontal lines represent the cutoff values.
IgG avidity ELISA with chimeric antigen MIC1-MAG1.
Fourteen serum samples from patients in the acute phase of toxoplasmosis and 14 serum samples from chronically infected individuals, namely, groups I and III, respectively, which reacted with chimeric antigen MIC1-MAG1 were examined in an IgG avidity ELISA with the use of 6 M and 8 M urea solutions. The avidities of the Igs directed against the epitopes carried by chimeric antigen MIC1-MAG1 were compared with the avidities measured by the lysed whole-cell assay (VIDAS TOXO IgG Avidity; bioMérieux). All 14 sera from patients with chronic toxoplasmosis reacted with high avidity, for both the tests using 6 M urea and those using 8 M urea (Table 2). Only one serum sample from an individual in the acute phase of infection reacted with low avidity, while three sera reacted with borderline avidity and 10 reacted with high avidity in the IgG ELISA utilizing 6 M urea. Examination of the same serum samples, namely, acute-phase toxoplasmosis but with the use of 8 M urea, showed that 9 sera reacted with low avidity, 2 reacted with borderline avidity, and 3 reacted with high avidity.
Table 2.
Comparison of a commercial avidity test using TLA and an in-house ELISA with the MIC1-MAG1 chimeric antigen based on results for 28 sera from patients in the acute or chronic phase of T. gondii infection
| Serum sample groupa and antigen | No. (%) of samplesb |
Mean AI (range) | ||
|---|---|---|---|---|
| Low AI | Borderline AI | High AI | ||
| Acute phase of toxoplasmosis | ||||
| TLA (VIDAS) | 14 (100) | 0 | 0 | 0.13 (0.11–0.15) |
| MIC1-MAG1, 6 M urea | 1 (7) | 3 (21.5) | 10 (71.5) | 0.43 (0.12–0.63) |
| MIC1-MAG1, 8 M urea | 9 (64.5) | 2 (14) | 3 (21.5) | 0.30 (0.10–0.56) |
| Chronic phase of toxoplasmosis | ||||
| TLA (VIDAS) | 0 | 0 | 14 (100) | 0.52 (0.44–0.65) |
| MIC1-MAG1, 6 M urea | 0 | 0 | 14 (100) | 0.71 (0.56–0.86) |
| MIC1-MAG1, 8 M urea | 0 | 0 | 14 (100) | 0.55 (0.44–0.76) |
Fourteen serum samples from each group were examined.
Shown is the number of serum samples that reacted. For TLA (VIDAS), an AI below 0.2 is low, an AI of 0.2 to 0.3 is borderline, and an AI over 0.3 is high; for MIC1-MAG1, an AI below 0.3 is low, an AI of 0.3 to 0.4 is borderline, and an AI over 0.4 is high.
DISCUSSION
The assays for the diagnosis of toxoplasmosis which are currently available rely primarily on the use of either live or chemically treated tachyzoites or extracts of whole tachyzoites grown in mice and/or tissue culture. Whole-parasite extracts are complex mixtures of native antigens. The methods of producing these antigens may vary significantly between laboratories. It is known that the antigen(s) obtained from tachyzoites may contain various nonparasitic materials from the culture medium and eukaryotic host cells. Thus, serological tests based on tachyzoite antigenic extracts are difficult to standardize. One approach to the improvement of these tests is to replace the native antigens with recombinant proteins. In the past 20 years, numerous recombinant antigens of T. gondii have been produced in bacterial and eukaryotic expression systems and evaluated for their potential as diagnostic antigens for the detection of specific antibodies in human serum samples. Several previous studies have found that recombinant antigens improve the serological diagnosis of toxoplasmosis (1–20, 22, 24–26, 29–30). The groups of antigens tested include dense granule proteins (rGRA1, rGRA2, rGRA4, rGRA5, rGRA6, rGRA7, and rGRA8), rhoptry antigens (rROP1 and rROP1), microneme proteins (rMIC1, rMIC2, rMIC3, rMIC4, and rMIC5), surface antigens (rSAG1, rSAG2, and P35), and matrix antigen protein rMAG1. These recombinant proteins are used mainly as antigens in ELISAs for the detection of specific IgG and IgM antibodies and are usually used singly to coat ELISA plates. However, combinations of recombinant antigens have been shown to be more sensitive than single antigens in detecting toxoplasmosis (14). A mixture of GRA7, GRA8, and ROP1 has previously been suggested for the detection of IgM antibodies (1), whereas several mixtures of recombinant proteins, namely, GRA7, GRA8, and SAG1 (1); GRA7, GRA8, SAG2, and H4 (19); SAG1, GRA1, and GRA7 (26); P35 (GRA8), SAG2, and GRA6 or MIC1ex2, MAG1, and MIC3 (11); and MAG1, SAG1, and GRA5 or GRA2, SAG1, and GRA5, or ROP1, SAG1, and GRA5 (14), have been proposed for the detection of IgG antibodies. Nevertheless, although they are promising, none of the assays based on recombinant proteins have displayed all of the characteristics required to replace the TLA in IgG- and IgM-based tests, indicating that further work is needed before an immunoassay with recombinant products becomes available for clinical purposes. For these reasons, the aim of this study was to improve the performance of the IgG ELISA on the basis of a new recombinant product, chimeric antigens.
This study evaluated the diagnostic utility of the new MIC1-MAG1 chimeric antigen for the serological diagnosis of toxoplasmosis in humans for the first time. Two fragments of the T. gondii MIC1 and MAG1 proteins were used to construct the chimeric antigen. The N-terminal part of MIC1, encoded by exon 2 of the mic1 gene, and a fragment of the MAG1 protein were chosen, in line with previous results that have shown the highly reactive potential of these protein regions in an IgG ELISA (11, 12). Pure, immunologically active chimeric antigenic protein was obtained in quantities of 43 mg/liter of culture using a very efficient E. coli expression system and a simple purification method. The diagnostic value of the MIC1-MAG1 chimeric antigen was evaluated with the Western blotting and IgG ELISA techniques. One hundred ten sera from patients in the acute, postacute, and chronic phases of toxoplasmosis were tested. This study demonstrates that, for all of the positive sera, the sensitivity of the IgG ELISA for the MIC1-MAG1 chimeric antigen was almost as high as that for the TLA (90.9% and 91.8%, respectively). However, the IgG antibodies in the sera from patients in the acute phase of toxoplasmosis (IgM positive, IgG with low avidity), as well as from individuals in the postacute phase of disease (IgM negative, IgG with low avidity), reacted more strongly with the chimeric antigen (100%) than with the TLA (just over 88%). Examination of the group III sera (from patients with chronic toxoplasmosis [IgM negative, IgG with high avidity]) showed lower reactivity with MIC1-MAG1 (85.1%) than with the TLA (94.0%). However, the reactivity of the chimeric protein with the IgG antibodies from the chronic-phase sera was definitely higher than that of the recombinant antigen combination (65.7%). A more precise analysis of this group of serum samples showed that the sensitivity of the IgG ELISA with the chimeric antigen is still high, at 100%, for sera from subgroups IIIA and IIIB, namely, individuals with titers of >50 IU/ml, and decreased, at 50%, for the sera from patients with low IgG titers of <50 IU/ml (subgroup IIIC). Furthermore, the mean absorbance values calculated for groups I, II, and III of sera showed that the chimeric product presents higher reactivity than the single antigens (rMIC1ex2 and rMAG1) or even a mixture of the two proteins. The high reactivity of the MIC1-MAG1 chimeric antigen can be explained by the fact that this construct contains the fragments of two proteins, MAG1, which has been suggested to be a molecular marker of the acute phase of toxoplasmosis (12), and MIC1ex2, which reacted strongly with sera from patients in the acute phase and slightly less strongly with sera from patients in the chronic phase of disease (11). Moreover, the higher reactivity of the chimeric antigen than the mixture of recombinant proteins (rMAG1 and rMIC1ex2) may be explained by the fact that the epitopes of this chimeric construct are probably more exposed to the anti-T. gondii antibodies. At the same time, a steric obstruction between molecules may occur in the case of a combination of recombinant antigens.
This study also evaluated the usefulness of MIC1-MAG1 in an IgG avidity assay on the basis of results obtained with 28 sera from patients infected with T. gondii. It demonstrated that all of the sera from patients with chronic toxoplasmosis were identified by high avidity using not only a commercial test but also an ELISA employing the chimeric antigen (for both ELISA with 6 and 8 M urea). Examination of the sera from patients in the acute phase of infection showed more varied results with respect to the AIs. This study shows that, in an IgG avidity ELISA using 6 M urea, only one serum sample reacted with low avidity, but for the assay using 8 M urea, the number of sera reacting with low avidity increased to nine. On the basis of these results, this study demonstrates that the maturation of the avidity of IgG antibodies for the chimeric antigen differed from that of the IgG antibodies for TLA. However, the IgG avidity ELISA using 8 M urea buffer and the MIC1-MAG1 chimeric antigen seems to have a potential importance in differentiating the acute from the chronic phase of infection. Nevertheless, more sera should be examined, with the aim of comparative testing of the maturation of antibody avidity for the chimeric antigen and the TLA in sera from patients where the duration of T. gondii infection is known.
To summarize, this report presents results which demonstrate, for the first time, that the use of the new MIC1-MAG1 chimeric antigen in an IgG ELISA is characterized by a sensitivity higher than that obtained with the TLA. Therefore, this recombinant protein may constitute a useful tool in the diagnosis of toxoplasmosis, giving far better results than a mixture of antigens. Additionally, the use of the MIC1-MAG1 protein in an IgG avidity ELISA may be useful in discriminating between phases of toxoplasmosis. However, despite the promising results presented here, more assays are required before MIC1-MAG1 can be used for clinical purposes.
ACKNOWLEDGMENT
This study was supported by Polish State Committee for Scientific Research grant N302 130438 to L.H.-G.
Footnotes
Published ahead of print 23 November 2011
REFERENCES
- 1. Aubert D, et al. 2000. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J. Clin. Microbiol. 38:1144–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beghetto E, et al. 2003. Use of an immunoglobulin G avidity assay based on recombinant antigens for diagnosis of primary Toxoplasma gondii infection during pregnancy. J. Clin. Microbiol. 41:5414–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beghetto E, Spadoni A, Bruno L, Buffolano W, Gargano N. 2006. Chimeric antigens of Toxoplasma gondii: toward standardization of toxoplasmosis serodiagnosis using recombinant products. J. Clin. Microbiol. 44:2133–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Béla SR, et al. 2008. Use of SAG2A recombinant Toxoplasma gondii surface antigen as a diagnostic marker for human acute toxoplasmosis: analysis of titers and avidity of IgG and IgG1 antibodies. Diagn. Microbiol. Infect. Dis. 62:245–254 [DOI] [PubMed] [Google Scholar]
- 5. Buffolano W, et al. 2005. Use of recombinant antigens for early postnatal diagnosis of congenital toxoplasmosis. J. Clin. Microbiol. 43:5916–5924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cong H, et al. 2008. Multi-epitope DNA vaccine linked to the A2/B subunit of cholera toxin protect mice against Toxoplasma gondii. Vaccine 26:3913–3921 [DOI] [PubMed] [Google Scholar]
- 7. Golkar M, et al. 2008. Serodiagnosis of recently acquired Toxoplasma gondii infection in pregnant women using enzyme-linked immunosorbent assays with a recombinant dense granule GRA6 protein. Diagn. Microbiol. Infect. Dis. 61:31–39 [DOI] [PubMed] [Google Scholar]
- 8. Golkar M, et al. 2007. The dense granule protein GRA2, a new marker for serodiagnosis of acute Toxoplasma infection: comparison of sera collected in both France and Iran from pregnant women. Diagn. Microbiol. Infect. Dis. 58:419–426 [DOI] [PubMed] [Google Scholar]
- 9. Harning D, Spenter J, Metsis A, Vuust J, Petersen E. 1996. Recombinant Toxoplasma gondii surface antigen 1 (P30) expressed in Escherichia coli is recognized by human Toxoplasma-specific immunoglobulin M (IgM) and IgG antibodies. Clin. Diagn. Lab. Immunol. 3:355–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hiszczyńska-Sawicka E, et al. 2005. Efficient production of the Toxoplasma gondii GRA6, p35 and SAG2 recombinant antigens and their applications in serodiagnosis of toxoplasmosis. Acta Parasitol. 50:249–254 [Google Scholar]
- 11. Holec L, Gąsior A, Brillowska-Dąbrowska A, Kur J. 2008. Toxoplasma gondii: enzyme-linked immunosorbent assay using different fragments of recombinant microneme protein 1 (MIC1) for detection of immunoglobulin G antibodies. Exp. Parasitol. 119:1–6 [DOI] [PubMed] [Google Scholar]
- 12. Holec L, Hiszczyńska-Sawicka E, Gąsior A, Brillowska-Dąbrowska A, Kur J. 2007. Use of MAG1 recombinant antigen for diagnosis of Toxoplasma gondii infection in humans. Clin. Vaccine Immunol. 14:220–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holec-Gasior L, Drapała D, Lautenbach D, Kur J. 2010. Toxoplasma gondii: usefulness of ROP1 recombinant antigen in an immunoglobulin G avidity assay for diagnosis of acute toxoplasmosis in humans. Pol. J. Microbiol. 59:307–310 [PubMed] [Google Scholar]
- 14. Holec-Gasior L, Kur J. 2010. Toxoplasma gondii: recombinant GRA5 antigen for detection of immunoglobulin G antibodies using enzyme-linked immunosorbent assay. Exp. Parasitol. 124:272–278 [DOI] [PubMed] [Google Scholar]
- 15. Holec-Gasior L, Kur J, Hiszczyńska-Sawicka E. 2009. GRA2 and ROP1 recombinant antigens as potential markers for detection of Toxoplasma gondii-specific immunoglobulin G in humans with acute toxoplasmosis. Clin. Vaccine Immunol. 16:510–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacobs D, Vercammen M, Saman E. 1999. Evaluation of recombinant dense granule antigen 7 (GRA7) of Toxoplasma gondii for detection of immunoglobulin G antibodies and analysis of a major antigenic domain. Clin. Diagn. Lab. Immunol. 6:24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lau YL, Fong MY. 2008. Toxoplasma gondii: serological characterization and immunogenicity of recombinant surface antigen 2 (SAG2) expressed in the yeast Pichia pastoris. Exp. Parasitol. 119:373–378 [DOI] [PubMed] [Google Scholar]
- 18. Lecordier L, et al. 2000. Enzyme-linked immunosorbent assays using the recombinant dense granule antigens GRA6 and GRA1 of Toxoplasma gondii for detection of immunoglobulin G antibodies. Clin. Diagn. Lab. Immunol. 7:607–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li S, et al. 2000. Serodiagnosis of recently acquired Toxoplasma gondii infection using an enzyme-linked immunosorbent assay with a combination of recombinant antigens. Clin. Diagn. Lab. Immunol. 7:781–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li S, et al. 2000. Serodiagnosis of recently acquired Toxoplasma gondii infection with a recombinant antigen. J. Clin. Microbiol. 38:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liesenfeld O, et al. 2001. Confirmatory serologic testing for acute toxoplasmosis and rate of induced abortions among women reported to have positive Toxoplasma immunoglobulin M antibody titers. Am. J. Obstet. Gynecol. 184:140–145 [DOI] [PubMed] [Google Scholar]
- 22. Martin V, et al. 1998. Detection of human Toxoplasma-specific immunoglobulins A, M, and G with a recombinant Toxoplasma gondii Rop2 protein. Clin. Diagn. Lab. Immunol. 5:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montoya JG. 2002. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J. Infect. Dis. 185(Suppl. 1):S73–S82 [DOI] [PubMed] [Google Scholar]
- 24. Nigro M, et al. 2003. Evaluation of Toxoplasma gondii recombinant proteins for diagnosis of recently acquired toxoplasmosis by an immunoglobulin G analysis. Diagn. Microbiol. Infect. Dis. 47:609–613 [DOI] [PubMed] [Google Scholar]
- 25. Pfrepper KI, et al. 2005. Seroreactivity to and avidity for recombinant antigens in toxoplasmosis. Clin. Diagn. Lab. Immunol. 12:977–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pietkiewicz H, et al. 2004. Usefulness of Toxoplasma gondii-specific recombinant antigen in serodiagnosis of human toxoplasmosis. J. Clin. Microbiol. 42:1779–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pietkiewicz H, et al. 2007. Usefulness of Toxoplasma gondii recombinant antigens (GRA1, GRA7, SAG1) in an immunoglobulin G avidity test for serodiagnosis of toxoplasmosis. Parasitol. Res. 100:333–337 [DOI] [PubMed] [Google Scholar]
- 28. Suzuki LA, Rocha RJ, Rossi CL. 2001. Evaluation of serological markers for the immunodiagnosis of acute acquired toxoplasmosis. J. Med. Microbiol. 50:62–70 [DOI] [PubMed] [Google Scholar]
- 29. Suzuki Y, et al. 2000. Detection of immunoglobulin M antibodies to P35 antigen of Toxoplasma gondii for serodiagnosis of recently acquired infection in pregnant women. J. Clin. Microbiol. 38:3967–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu K, et al. 2009. Diagnosis of human toxoplasmosis by using the recombinant truncated surface antigen 1 of Toxoplasma gondii. Diagn. Microbiol. Infect. Dis. 64:261–266 [DOI] [PubMed] [Google Scholar]
- 31. Yang CD, Chang GN, Chao D. 2004. Protective immunity against Toxoplasma gondii in mice induced by a chimeric protein rSAG1/2. Parasitol. Res. 92:58–64 [DOI] [PubMed] [Google Scholar]