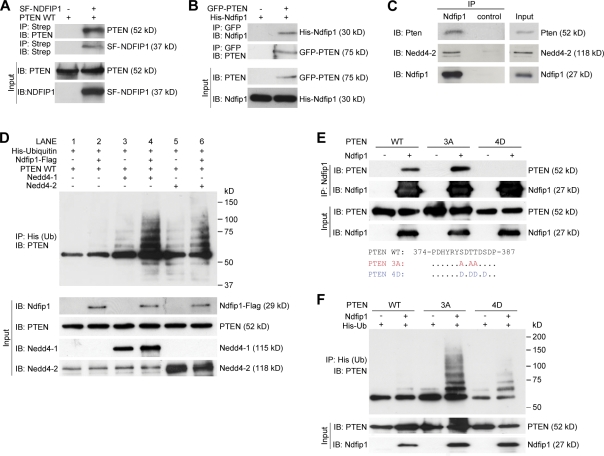
Figure 2.
Ndfip1 binds to PTEN and enhances PTEN ubiquitination by Nedd4 E3 ligases. (A) PTEN (PTEN WT) coprecipitates with Strep-Flag-NDFIP1 (SF-NDFIP1) after expression in HEK-293T cells. IB, immunoblotted; IP, immunoprecipitated. (B) Ndfip1 coprecipitates with GFP-PTEN after expression in HEK-293T cells. (C) Mouse cortical lysates show coimmunoprecipitation of both Pten and Nedd4-2 with Ndfip1. (D) PTEN ubiquitin (Ub) assay using Ndfip1, Nedd4-1, and Nedd4-2 in HEK-293T cells. Lanes without Ndfip1 coexpression (lanes 1, 3, and 5) show decreased PTEN ubiquitination compared with lanes expressing Ndfip1 (lanes 2, 4, and 6). The presence of Ndfip1 with Nedd4-1 (lane 4) or Nedd4-2 (lane 6) produced the characteristic monoubiquitinated ladder together with higher molecular mass–polyubiquitinated smear. (E) Phosphorylation status of PTEN changes Ndfip1 interaction. PTEN WT and the PTEN 3A phosphorylation-defective mutant interact strongly with Ndfip1, but PTEN 4D phosphomimic mutant interacts weakly with Ndfip1. (F) Ndfip1-mediated ubiquitination of PTEN is dependent on the phosphorylation status of PTEN. In the absence of Ndfip1, little PTEN ubiquitination occurs. Coexpression with Ndfip1 results in strong ubiquitination of the PTEN 3A phosphorylation-defective mutant, but both PTEN WT and the PTEN 4D phosphomimic mutant are weakly ubiquitinated.