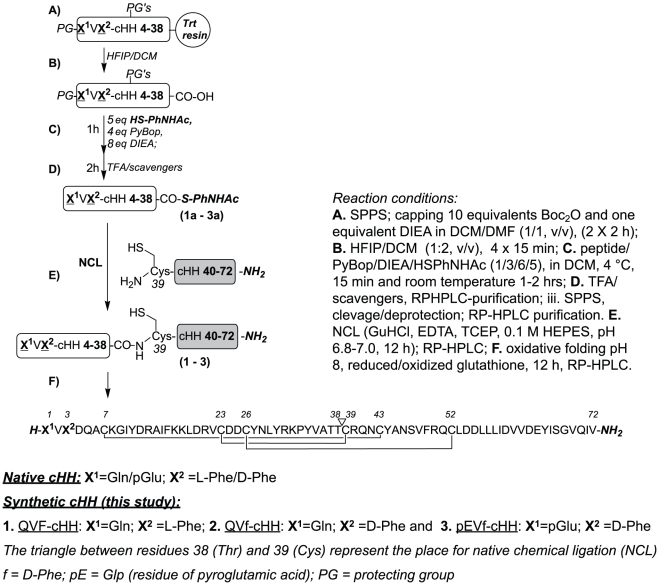
Figure 1. Scheme of the SPPS coupled to NCL to obtain the full length peptides.
The leading segments containing a C-terminal thioester, QVF-cHH4-38, QVdF-cHH4-38, pEVdF-cHH4-38, and the cHH39-72 following segment with a free N-terminal cysteine were synthesized by SPPS. The NCL reaction between the leading and following segments returned the full length 72mer cHH isomers, which were further subjected to oxidative folding to obtain the folded peptides.