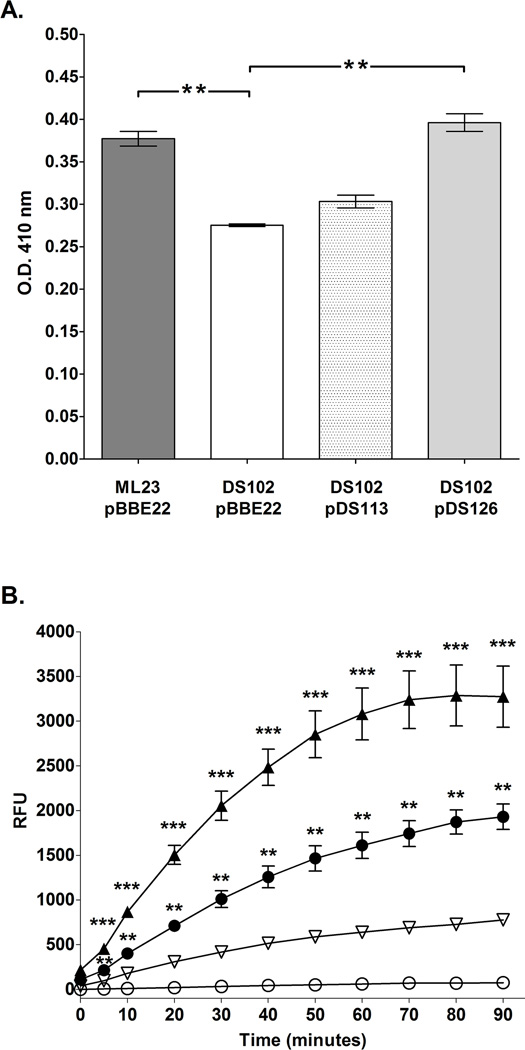
Figure 3.
BB0646 functions as a lipase with specificity for both saturated and polyunsaturated fatty acid substrates. (A) Whole cell lysates from ML23/pBBE22, DS102/pBBE22, DS102/pDS113 and DS102/pDS126 were assayed using p-nitrophenyl palmitic acid as a substrate. Note the statistically significant decrease in lipase activity for DS102/pBBE22 relative to the isogenic parent and the complement DS102/pDS126. The dual asterisks (**) denote a P value of < 0.01. (B) Whole cell lysates of either ML23/pBBE22, DS102/pBBE22, DS102/pDS113 or DS102/pDS126 were incubated with the fluorogenic substrate, 7-hydroxycoumarinyl linolenic acid over a period of 90 min. and read at 10 min. intervals. Note that the bb0646::gentR mutant (DS102/pBBE22; open circles) was nearly devoid of activity whereas the parent (ML23/pBBE22; closed circles) both complements (DS102/pDS113; inverted open triangles and DS102/pDS126; closed triangles) showed restoration of lipase activity with highest activity seen for DS102/pDS126. Bars indicate standard error. Time points from each strain were tested for significance; **, and *** denote P values < 0.001, and 0.0001 respectively.