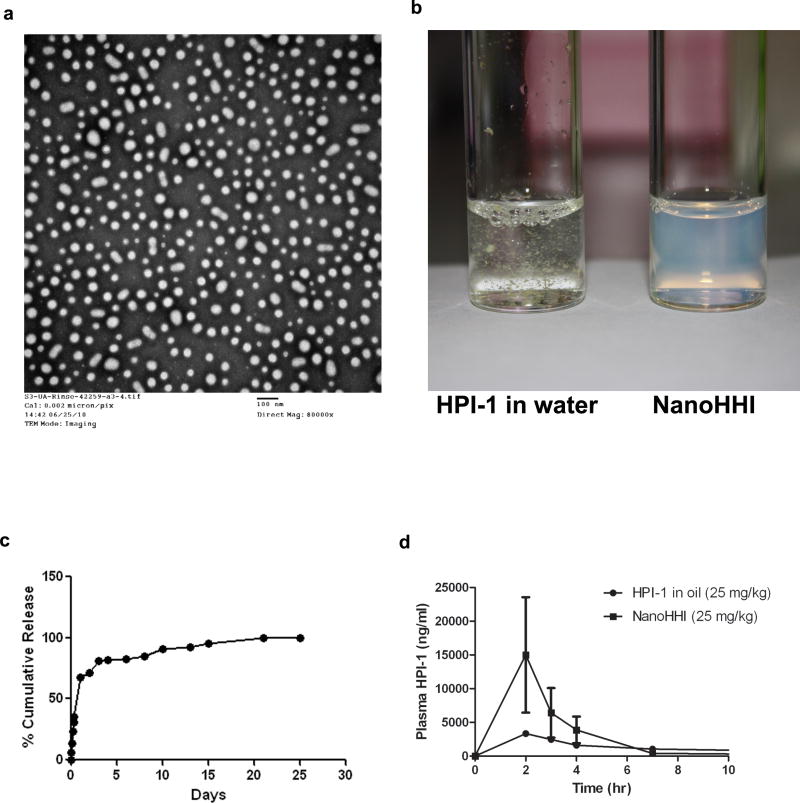
Figure 1. Physicochemical characterization and pharmacokinetics of NanoHHI.
(a) Transmission electron micrograph (TEM) pictures of NanoHHI, demonstrating uniformly dispersed nanoparticles with an average size diameter of <100nM. The scale bar on lower right corner equals 100nM.
(b) NanoHHI (right) is a uniform suspension in aqueous media while parental HPI-1 is essentially insoluble, with flakes of drug clearly visible in the suspension. Equivalent amounts of HPI-1 were used in both instances.
(c) In vitro release kinetics of HPI-1 from NanoHHI, performed at 37°C. The Y-axis charts the % cumulative release and X-axis denotes time in days.
(d) Pharmacokinetic disposition of parenteral NanoHHI administered as a single dose (25 mg/kg) compared to that of “free” HPI-1 (25 mg/kg) in corn oil. The experiments were performed in non-tumor bearing CD1 mice, with three mice per cohort. Plasma HPI-1 levels were assessed using LC-MS/MS.