Abstract
Root development is sensitive to environmental stimuli. We have recently reported that the light signal could promote the helical growth of seminal roots and drive the wavy root morphology in rice (Oryza sativa L.) young seedlings. The light-stimulated wavy roots were mostly performed in indica-type rice varieties (e.g., Taichung Native 1; TCN1) but not in japonica rice (e.g., Tainung 67; TNG67). Here, we demonstrated that the light-driven circumutation trajectory of TCN1 seminal roots could be changed if the seedling roots were grown in the medium containing high concentration of Phytagel. The data showed the root morphology would be modulated from wavy to curling when the Phytagel concentration was increased to 2%. However, the touch-stimulated curling root phenotype could not be performed in dark. In addition, the touch-induced curling roots were not appeared in the TNG67 rice cultivar. Although touch stimuli could not induce wavy/curling root phenotype in dark, it could modify the light-promoted helical growth to conduct curling roots in TCN1 rice seedlings. Thus, it was suggested that there is a crosstalk mechanism between touching-induced root curling and light-stimulated root waving.
Key words: curling root, light stimuli, Oryza sativa, seminal root, touch stimuli, wavy root
Root development and architecture could be changed to adapt the environmental conditions. Although root is usually grown in soil, it still exposes to light penetrated through soil particles. Some studies also indicated light can be conducted from shoots to roots through vascular bundle tissues.1,2 Recently, we have reported that the light-exposed seminal roots of indica-type rice, i.e., Taichung Native 1 (TCN1), presented the wavy morphology.3 The light-induced wavy root was not performed in japonica rice such as Tainung 67 (TNG67). Moreover, the circumutation of TCN1 seminal root tip were observed with time-lapse photography during root growth. According to the investigations among various rice varieties, it has been found that the root morphology was determined by helix period and circumnutation trajectory of root tip moving behavior.3 For example, the root tip movement of light-exposed TCN1 seedlings was a regular circumntation; therefore, the roots performed a regular wavy phenotype. In the other rice variety (i.e., Taichung Sen 17) with the curling root morphology, the circumnutation trajectory of seminal roots was significantly irregular compared with that was observed in TCN1. In the previous report, we showed that the auxin and oxylipins (i.e., ketol) played important roles to trigger the light-induced wavy roots.3
The wavy root phenotype has also been observed in Arabidopsis when it was cultured on an agar-plate that was inclined at an angle of less than 90°.4 Based on the studies in Arabidopsis mutants, the performance of obstacle-touching induced wavy phenotype in seedlings roots was related to the functions of auxin efflux/influx carriers and some proteins involved in cell expansion.4–6 Moreover, ethylene also played a role to modulate the wavy root morphology.7
In our previous experiments for studying the light-induced wavy roots, rice seedlings were cultured in water. In order to reveal the effect of interaction between light signal pathway and touch stimuli on rice seminal root growth, the sterilized rice seeds of TCN1 and TNG67 cultivars were germinated at 30°C in dark for 2 d and moved to continuous white light conditions (90 µmol m−2 s−1) to grow in vertically oriented square dishes containing 1.5% and 2% (w/v) Phytagel (Sigma, St. Louis, MO), respectively. The Phytagel percentage of the medium that we used here were higher than that was used for plant tissue culture in usual. After 3 d culture, the seminal roots of seedlings on 1.5% Phytagel performed wavy phenotype that was similar to the wavy roots observed in water-cultured seedlings under light conditions. Furthermore, the seminal roots in 2% Phytagel was grown to be a curling type (Fig. 1). On the other hand, no wavy or curling root morphology was presented in dark conditions either in 1.5% or 2% Phytagel-containing medium (Fig. 1). These results showed that root-Phytagel interaction could not directly induce the significant wavy or curling root morphology under dark growth conditions, but it could modify the light-stimulated helical growth and conduct the curling root morphology.
Figure 1.
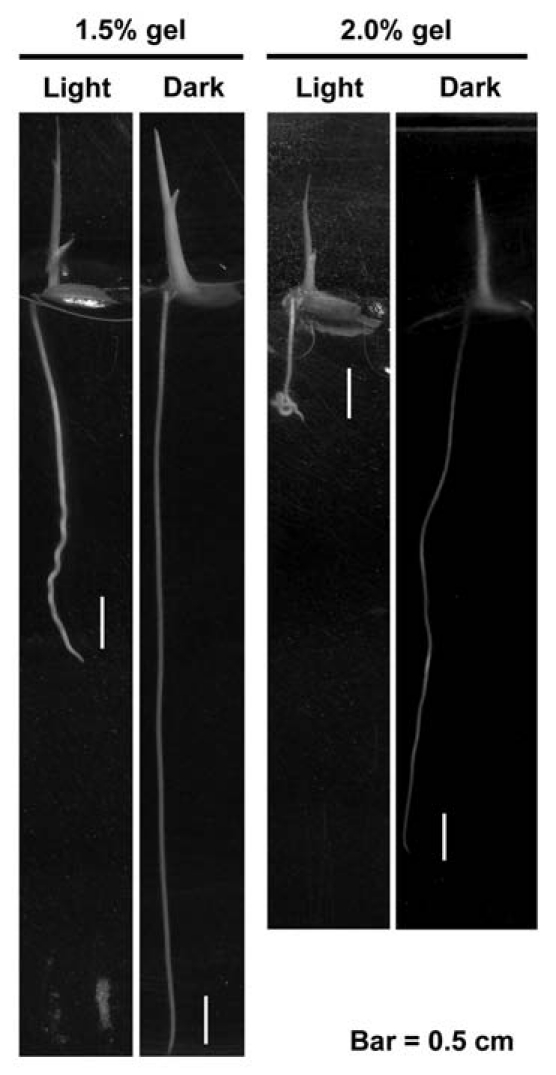
Effect of the interaction between light signals and touch stimuli on seminal root growth in rice seedlings. The TCN1 rice seeds were germinated in dark for 2 d and then germinated seeds were transferred to 1.5% and 2% Phytagel-containing plates for continuously growing. The root morphology was investigated after 3 d of Phytagelculture under light and dark conditions.
Photomorphology of the seminal roots was diverse among rice varieties. Our previous data showed light-induced wavy roots could not be conducted in TNG67 rice cultivar.3 Here, we also observed the root growth of TNG67 rice seedlings on Phytagel-containing plates, and the results showed the straight root morphology in both light and dark conditions (data not shown). These results indicated that the phenomena of touch-stimulated curling roots were also rice variety-dependent.
Based on above mentioned results, it was suggested that mechanisms of root-gel interaction for conducting curling phenotype was highly correlated with the transduction pathway of light signal to induce root waving. This hypothesis was supported by the observation on physiological mechanisms of light-induced wavy roots in rice plants and the obstacle-touching stimulated wavy roots in Arabidopsis. Our previous observation in rice plants suggested that auxin polar transport was essential for light-induced root waving and fatty acid oxygenation was involved to the mechanism of root waving in light.3 In Arabidopsis, auxin polar transport was also indicated to play a role in obstacle-touching stimulated root waving.8,9 In addition, wavy roots of Arabidopsis could be induced by several products of fatty acid oxygenation, i.e., ketols, ketones and hydroxides.10
In conclusion, both light signal and touch stimuli were the important environmental cues to guide root growth and determine root morphology. Touch stimuli were able to modify the trajectory of light-induced root waving. Phenomena of both light-induced wavy roots and touch-stimulated curling roots were rice variety-dependent. Furthermore, it was suggested that touch-induced signaling may be associated with the light-induced signal pathway to conduct curling phenotype in seminal roots of rice seedlings.
Acknowledgments
We would like to thank Dr. Chih-Sheng Sheu at Taichung District Agricultural Research and Extension Station in Taiwan for providing the rice seeds.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Mandoli DF, Briggs WR. The photoperceptive sites and the function of tissue light-piping in photomorphogenesis of etiolated oat seedlings. Plant Cell Environ. 1982;5:137–145. [Google Scholar]
- 2.Sun Q, Yoda K, Suzuki M, Suzuki H. Vascular tissue in the stem and roots of woody plants can conduct light. J Exp Bot. 2003;54:1627–1635. doi: 10.1093/jxb/erg167. [DOI] [PubMed] [Google Scholar]
- 3.Wang SJ, Ho CH, Chen HW. Rice develop wavy seminal roots in response to light stimulus. Plant Cell Rep. 2011;30:1747–1758. doi: 10.1007/s00299-011-1082-2. [DOI] [PubMed] [Google Scholar]
- 4.Okada K, Shimura Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science. 1990;250:274–276. doi: 10.1126/science.250.4978.274. [DOI] [PubMed] [Google Scholar]
- 5.Yuen CY, Pearlman RS, Silo-Suh L, Hilson P, Carroll KL, Masson PH. WVD2 and WDL1 modulate helical organ growth and anisotropic cell expansion in Arabidopsis. Plant Physiol. 2003;131:493–506. doi: 10.1104/pp.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abe T, Thitamadee S, Hashimoto T. Microtubule defects and cell morphogenesis in the lefty1lefty2 tubulin mutant of Arabidopsis thaliana. Plant Cell Physiol. 2004;45:211–220. doi: 10.1093/pcp/pch026. [DOI] [PubMed] [Google Scholar]
- 7.Buer CS, Wasteneys GO, Masle J. Ethylene modulates root-wave responses in Arabidopsis. Plant Physiol. 2003;132:1085–1096. doi: 10.1104/pp.102.019182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons C, Migliaccio F, Masson P, Caspar T, Soll D. A novel root gravitropism mutant of Arabidopsis thaliana exhibiting altered auxin physiology. Physiol Plant. 1995;93:790–798. doi: 10.1111/j.1399-3054.1995.tb05133.x. [DOI] [PubMed] [Google Scholar]
- 9.Santner AA, Watson JC. The WAG1 and WAG2 protein kinases negatively regulate root waving in Arabidopsis. Plant J. 2006;45:752–764. doi: 10.1111/j.1365-313X.2005.02641.x. [DOI] [PubMed] [Google Scholar]
- 10.Vellosillo T, Martínez M, López MA, Vicente J, Cascón T, Dolan L, et al. Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell. 2007;19:831–846. doi: 10.1105/tpc.106.046052. [DOI] [PMC free article] [PubMed] [Google Scholar]


