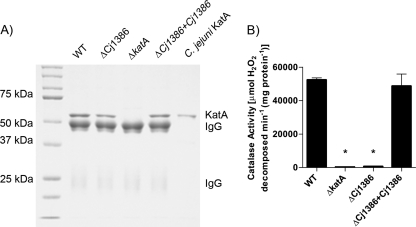
Fig 5.
KatA immunoprecipitated from the ΔCj1386 mutant has decreased catalase activity relative to wild-type C. jejuni. (A) Immunoprecipitated KatA from wild-type C. jejuni, ΔCj1386, ΔkatA, and ΔCj1386+Cj1386 strains eluted in 50 mM glycine, pH 2.8. Five microliters of each immunoprecipitated sample and 0.5 μg of purified C. jejuni KatA were loaded into each lane and separated on a 10% denaturing SDS-PAGE gel. (B) Catalase activity of KatA immunoprecipitated samples eluted in soft-elution buffer from wild-type C. jejuni, ΔCj1386, ΔkatA, and ΔCj1386+Cj1386 strains. KatA protein concentrations were determined by densitometry from the SDS-PAGE gel (not shown), and 250 μg of KatA was assayed for activity. Catalase activity is expressed as μmol of hydrogen peroxide decomposed per minute per mg of protein. Error bars indicate standard errors of the means (n = 4). An asterisk indicates a P value of <0.05 using the Student t test.