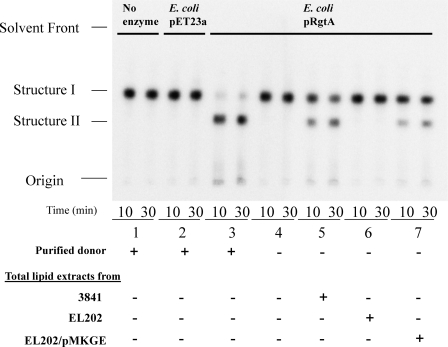
FIGURE 3.
RgtA activity assay with total lipid extracts. Total lipids were tested for their ability to serve as a GalA donor in an activity assay with RgtA overexpressed in E. coli membranes and the radiolabeled acceptor substrate 1-dephospho-[4′-32P]lipid IVa (Structure I). Standard reaction mixtures are described under “Experimental Procedures.” Products were separated by thin layer chromatography and viewed by a radio imager. Lanes 1 and 2 lack enzyme. Lanes 3–7 contain, as an enzyme source, membranes from E. coli overexpressing RgtA. Lipid substrate was added where indicated by (+). The product in lane 3 was identified by Kanjilal-Kolar et al. (3, 4) to be GalA-modified 1-dephospho-[4′-32P]lipid IVa (Structure II). Conversion of substrate was not observed from reactions containing EL202 lipids (lane 6), indicating the lack of the necessary lipid donor (Dod-P-GalA) required for activity. Lipids from EL202 complemented with the rgtE gene (EL202/pMKGE) were able to recover RgtA activity (lane 7).