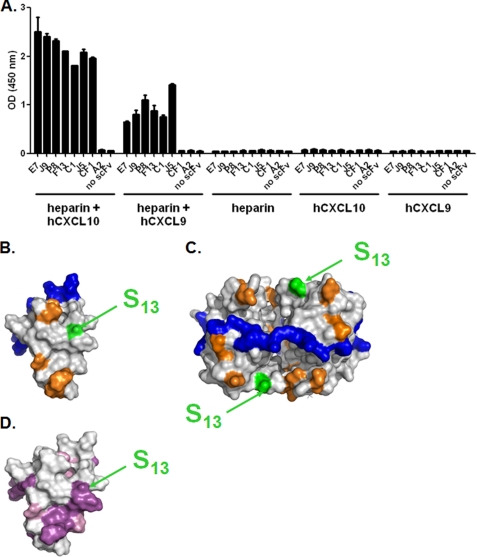
FIGURE 4.
Epitope accessibility in the context of glycosaminoglycans. A, E7, J9, P8, F13, C1, and J5 dual-specific scFvs were immobilized in an ELISA-based assay and tested for their capacity to bind to either hCXCL10 or hCXCL9 in complex to biotinylated heparin. scFv CF1 was added as a positive control for an anti-hCXCL10, and scFv A2 was added as an irrelevant negative control. Unspecific coating of either chemokine-heparin complex, chemokine, or heparin, in the absence of scFv, was also evaluated. Results are expressed as the mean ± S.D. of duplicates. B and C, Connolly surface representations of monomeric (B) hCXCL10 (Protein Data Bank 1LV9) and tetrameric (C) hCXCL10. Color representations are as follows: blue, GAG-interacting residues; orange, hCXCR3-interacting residues; green and arrows, Ser13. CXCR3-binding residues were determined according to Booth et al. (26). Potential GAG binding residues were suggested by Swaminathan et al. (22). D, Connolly surface representation of monomeric hCXCL10. Conserved residues between hCXCL9 and hCXCL10 were determined according to the alignment in Fig. 5F. Color representation is as follows: purple, conserved residues; pink, amino acid residues with similar physico-chemical properties according to BLOSUM 62 substitution matrix; arrows indicate Ser13.