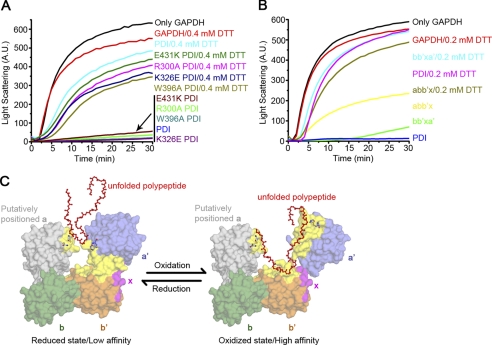
FIGURE 6.
Redox-regulated chaperone activity of hPDI. A and B, guanidine hydrochloride-denatured GAPDH at 0.14 mm was 50-fold diluted into refolding buffer in the absence or presence of 28 μm (A) or 5.6 μm (B) hPDI proteins with various concentrations of DTT as indicated. Aggregation produced during the refolding was monitored by recording the light scattering at 488 nm, and the suppression of aggregation was used to measure the chaperone activity of hPDI proteins. C, a schematic model of redox-regulated chaperone activity of hPDI. The b, b′, x, and a′ domains are colored in green, orange, magenta, and blue, respectively. The position of the a domain (gray) in this model is achieved by superposition of the bb′xa′ structure with that of yPDI at 4 °C (23). Residues involved in ligand binding (23, 34, 43, 44) are colored in yellow. Active sites of both the a and a′ domains are shown in stick representations. The reduced hPDI adopts the compact conformation with small hydrophobic areas exposed for substrate binding, and oxidation of the a′ domain of hPDI results in exposure of more extended hydrophobic areas for its substrate binding and chaperone activity.