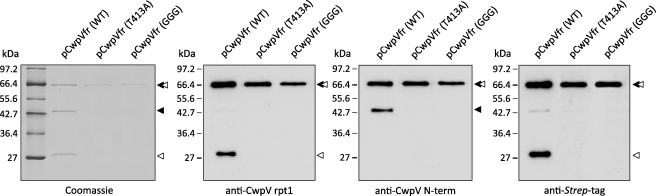
FIGURE 3.
CwpV undergoes enzyme-independent intramolecular autoprocessing. Plasmids encoding a Strep-tagged fragment of CwpV (pCwpVfr (WT), pCwpVfr(T413A), and pCwpVfr(GGG)) were used as templates in PURExpress in vitro protein synthesis reactions. Proteins of interest were purified on StrepTactin resin separated on 12% SDS-polyacrylamide gels and analyzed via Coomassie Blue staining and Western blotting. In the pCwpVfr (WT) sample, two products corresponding to the N-terminal domain and a fragment of the repeat domain could be seen aside from the full-length CwpV fragment. In both pCwpVfr(T413A) and pCwpVfr(GGG)samples, no cleavage was observed as only the full-length CwpV fragment could be seen. ◂, repeat domain; ◁, anchoring domain.