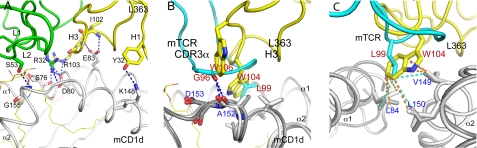
FIGURE 5.
L363 binding to mCD1d. A, L363 forms similar numbers of H-bonds including H-bonds to the same residues (Ser-76, Glu-83, and Asp-80) compared with mTCR. B, superimposing the structures of the L363-C20:2-αGalCer-mCD1d and Vα14Vβ8.2 TCR-C20:2-αGalCer-mCD1d complexes indicate that Trp-106 (W106) and Trp-104 (W104) of L363 H3 mimic residues Gly-96 (G96) and Leu-99 (L99) of TCR CDR3α loop, because they form similar contacts with mCD1d residues above the F′ pocket. Cyan, TCR Vα chain; yellow, L363 Fab VH chain; gray, mCD1d. C, Fab CDR3 VH residue Trp-104 and TCR CDR3α residue Leu-99 interact closely with the hydrophobic residues Leu-84, Val-149, and Leu-150, which form the F′ roof.