Abstract
Background
Carcinomas of the renal pelvis and ureter are rare diseases, accounting for only about 1% of all urogenital malignancies. Previous reports suggest that squamous cell histology is associated with inferior survival. We present the largest population based analysis to date of survival in patients with upper urinary tract malignancies.
Methods
We analyzed the Surveillance, Epidemiology and End Results database for cancer specific survival rates in patients with renal pelvis and ureteral malignancies who were diagnosed between 1973 and 2003 in the SEER catchment geographic areas. The primary exposure of interest was the underlying histology, squamous cell versus transitional cell differentiation. We performed descriptive statistics, non parametric survival analysis, and cox proportional hazard analysis.
Results
We identified 13,213 eligible patients, 7,716 renal pelvis and 5,497 ureteral carcinomas. Among this cohort, 179 patients had squamous cell carcinoma (SCC), 12,395 had transitional cell carcinoma (TCC), including 121 papillary, and 619 had other histologies. Overall, patients with SCC histology fared worse. The median overall survival time was 10 months for SCC and 63 months for TCC. The cox analysis revealed a HR 3.7 (95% CI 3.0–4.5) for SCC when compared to TCC and corrected for decade of diagnosis, age, gender, prior treatment, and race. The difference between the two groups was entirely attributable to survival differences in patients with loco-regional disease. However, when stratified by lymph node involvement this difference disappeared for patients with locally involved lymph nodes (P = 0.84) and for patients with clear lymph nodes (P = 0.92).
Conclusions
SCCs of the upper urinary tract present at a higher clinical stage and appear to represent more aggressive disease when compared to other histologies. However, when appropriately staged according to lymph node status, the survival of TCC and SCC of the upper urinary tract is identical when compared stage by stage.
Keywords: ureter, renal pelvis, squamous cell carcinoma, transitional carcinoma, survival analysis
Introduction
Neoplasms of the upper urogenital tracts involving the renal pelvis and ureter are defined as any neoplastic growth involving the lining of the urinary tract from the renal calyces to the ureterovesical junction.1 These tumors are rare representing less than one percent of genitourinary malignancies.2,3 The incidence of neoplasms of the upper urinary tract in the United States has increased slightly over the last 20 years from an annual incidence of 0.69 to 0.73/1000,000 person years from 1973 to 1996.4 The paucity of cases makes it difficult to establish a standard of care for these malignancies and the prognosis remains poor. Squamous cell histology represents only a small fraction of the total number of malignancies of the upper urinary tract (UUT). Most of our knowledge about these rare malignancies originates from anecdotal evidence and squamous cell histology, in particular, has been associated with a poor prognosis.5–10 A recent retrospective registry study from Sweden challenged this assumption.11 We present the largest registry of UUT malignancies to date and investigate the impact of squamous cell histology on overall survival and cancer specific survival.
Patient and Methods
The database
For our cohort we utilized publicly available data from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2004. The SEER program is an effort by the United States (US) government to collect data and report cancer incidence, treatment, and survival. The database represents about 10 percent of the US population by collecting information from 14 population based cancer registries around the country. Trained abstractors collect data on patient age, sex, site, histological findings, tumor grade, and stage from hospital records, outpatient clinics, radiation centers, private laboratories and physician offices. For coding purposes the ICD-O-3 classification was used.12
Patients
Patients were eligible if they presented between 1973 and 2004 with a malignancy of the renal pelvis or ureter (site coding C65.9 and C66.9). To allow for sufficient maturation, only patients presenting until the end of 2003 were included in the survival analysis. Only patients with a clearly malignant coded phenotype were included. The primary exposure of interest was the underlying histology. Histologies were subdivided into squamous cell (SC), transitional cell (TC), (including the papillary subtype) and other histologies (OT) (mainly small cell, sarcomatoid and undifferentiated carcinomas). The primary outcome of interest was cancer specific survival. We also examined overall survival and performed sensitivity analyses, excluding and right censoring patients who died from causes other than UUT malignancies. Since survival prognosticators in UUT malignancies are poorly delineated, we examined race, prior surgery and radiotherapy, decade of diagnosis, age at diagnosis, gender, preceding malignancies and stage as potential predictors of the primary outcome and cancer specific survival. Race was coded as Caucasian, Black, Hispanic or Asian/other. The other category included mainly smaller Asian subpopulations and the indigenous population of the Western hemisphere. Decade of diagnosis was coded into the 70s, 80s, 90s and 2000s, while the age group was subdivided into 49 or younger, 50 to 75, and older than 75 years of age at diagnosis. Prior therapies were classified as having been received or not. Secondary to coding issues, we utilized the traditional American Joint Committee on Cancer (AJCC) staging classification, which entails the following stages: in situ, local, regional and distant. The regional stage included patients with extension beyond the ureter or renal pelvis and patients with documented lymph node involvement.
Statistical methods
We examined descriptive statistics to describe demographic composition of the study population and distribution of the other relevant predictors of the outcome. Uni-variate Kaplan Meier survival analysis was performed to investigate which variables were of predictive relevance. In our cancer specific survival analysis, the patients who died from a cause other than a malignancy of the upper urinary tract were right censored.
We examined race, prior surgery and radiotherapy, decade of diagnosis, age at diagnosis, gender, preceding malignancies and stage as other potential predictors of outcome and retained them in our final Cox proportional hazard (Cox PH) model if they had a significant impact on survival on univariate, non parametric analysis. Differences along the strata of predictors of interest were evaluated using a log rank test. If the log rank test was statistically significant, the covariates were retained in the final model. In the final model, we performed Cox proportional hazards regression to determine hazard ratios.13 Post-estimation diagnostics were performed to analyze if the proportional hazard assumptions were violated, using graphical and computational methods.14,15 All analyses were performed using STATA 9.0 (College Station, TX).
Results
We identified 13,213 patients with malignancies of the UUT. One hundred and ninety nine patients had squamous cell malignancies of the UUT and 12,395 had transitional or papillary histology. Overall most patients were of Caucasian origin, although relatively more patients with squamous cell carcinomas were of Asian ethnicity. Most patients underwent surgery and only a small fraction of patients obtained radiation as part of their treatment. Interesting is that only a small fraction of patients with locoregional disease obtained appropriate lymph node sampling and recording. Most patients with loco-regional disease had an unknown loco-regional lymph nodes status. Eighty eight (88.0%) patients with squamous cell histology and 3,624 (84.8%) with transitional cell histology and loco-regional disease had no appropriate recording of their loco-regional lymph node status. Baseline characteristics by histology are displayed in Table 1. Transitional carcinomas and other histologies demonstrated male gender predominance, while the gender distribution for squamous cell carcinomas was equally balanced. Appropriate lymph node sampling in surgically resected cases was consistently reported in less than twenty percent, an observation that has persisted into the 21st century. Patients with squamous cell malignancies were more likely to present with regional (P < 0.01) and distant (P < 0.01) disease, while TCC had a higher likelihood to present at a local (P < 0.01) or in situ stage (P < 0.01). Patients with squamous cell histology were less likely to undergo radiation therapy (P < 0.01) and surgery (P < 0.01).
Table 1.
| Squamous histology | Transitional and papillary histology | Other histologies | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Renal pelvis | Ureter | Both sites | Renal pelvis | Ureter | Both sites | Renal pelvis | Ureter | Both sites | |
| Gender | |||||||||
| Men | 56 (46.3%) | 42 (53.9%) | 98 (49.3%) | 4396 (60.4%) | 3377 (66.0%) | 7,773 (62.7%) | 190 (60.1%) | 192 (63.3%) | 382 (61.7%) |
| Women | 65 (53.7%) | 36 (46.1%) | 101 (50.7%) | 2883 (39.6%) | 1739 (34.0%) | 4,622 (37.3%) | 126 (39.9%) | 111 (36.6%) | 237 (38.3%) |
| Race | |||||||||
| Caucasian | 102 (84.3%) | 62 (79.5%) | 164 (82.4%) | 6376 (87.6%) | 4574 (89.4%) | 10,950 (88.3%) | 260 (82.3) | 264 (87.1%) | 524 (84.6%) |
| Blacks | 7 (5.8%) | 5 (6.4%) | 12 (6.0%) | 308 (4.2%) | 131 (2.6%) | 439 (3.5%) | 24 (7.6%) | 19 (6.3%) | 43 (7.0%) |
| Hispanics | 3 (2.5%) | 4 (5.1%) | 7 (3.5%) | 200 (2.8%) | 117 (2.3%) | 317 (2.6%) | 16 (5.0%) | 5 (1.7%) | 21 (3.4%) |
| Asian/other | 9 (7.4%) | 7 (8.9%) | 16 (8.1%) | 395 (5.4%) | 294 (5.8%) | 689 (5.6%) | 16 (5.0%) | 15 (5.0%) | 31 (5.0%) |
| Age group | |||||||||
| 30–49 years old | 11 (9.1%) | 4 (5.1%) | 15 (7.5%) | 438 (6.0%) | 166 (3.2%) | 604 (4.9%) | 42 (13.3%) | 17 (5.6%) | 59 (9.5%) |
| 50–74 years old | 60 (49.6%) | 51 (65.4%) | 111 (55.8%) | 4199 (57.7%) | 2945 (57.6) | 7,144 (57.6%) | 174 (55.0%) | 155 (51.2%) | 329 (53.2%) |
| >75 years old | 50 (41.3%) | 23 (29.5%) | 73 (36.7%) | 2642 (36.3%) | 2005 (39.2%) | 4,647 (37.5%) | 100 (31.7%) | 131 (43.23%) | 231 (37.3%) |
| Decade of diagnosis | |||||||||
| 1973–1979 | 35 (28.9%) | 16 (20.5%) | 51 (25.6%) | 1251 (17.2%) | 751 (14.7%) | 2,002 (16.2%) | 82 (25.9%) | 53 (17.5%) | 135 (21.8%) |
| 1980–1989 | 39 (32.2%) | 30 (38.5%) | 69 (34.7%) | 2142 (29.4%) | 1486 (29.0%) | 3,628 (29.3%) | 92 (29.1%) | 103 (34.0%) | 195 (31.5%) |
| 1990–1999 | 34 (28.1%) | 21 (26.9%) | 55 (27.6%) | 2524 (34.7%) | 1836 (35.9%) | 4,360 (35.2%) | 96 (30.4%) | 95 (31.4%) | 191 (30.9%) |
| 2000–2004 | 13 (10.7%) | 11 (14.1%) | 24 (12.1%) | 1362 (18.7%) | 1043 (20.4%) | 2,405 (19.4%) | 46 (14.6%) | 52 (177.2%) | 98 (15.8%) |
| Stage | |||||||||
| In situ | 1 (0.8%) | 3 (3.9%) | 4 (2.0%) | 1158 (15.9%) | 1261 (24.7%) | 2,419 (19.5%) | 16 (5.0%) | 102 (33.7%) | 118 (19.1%) |
| Local | 14 (11.6%) | 15 (19.2%) | 29 (14.6%) | 2502 (34.4%) | 1757 (34.3%) | 4,259 (34.4%) | 85 (26.9%) | 39 (12.9%) | 124 (20.0%) |
| Regional | 56 (46.3%) | 44 (56.4%) | 100 (50.3%) | 2666 (36.6%) | 1606 (31.4%) | 4,272 (34.5%) | 78 (24.7%) | 49 (16.2%) | 127 (20.5%) |
| *-LN+ | 5 (8.9%) | 2 (4.5%) | 7 (7.0%) | 195 (7.3%) | 89 (5.5%) | 284 (6.7%) | 10 (12.8%) | 3 (6.1%) | 13 (10.2%) |
| *-LN− | 4 (7.1%) | 1 (2.3%) | 5 (5.0%) | 222 (8.3%) | 142 (8.8%) | 364 (8.5%) | 9 (11.5%) | 2 (4.1%) | 11 (8.7%) |
| *-Not available | 47 (83.9%) | (93.2%) | 88 (88.0%) | 2,249 (84.4%) | 1,375 (85.6%) | 3,624 (84.8%) | 59 (75.6%) | 44 (89.8% | 103 (81.1%) |
| Distal | 40 (33.1%) | 13 (16.7%) | 53 (26.6%) | 650 (8.9%) | 260 (5.0%) | 910 (7.3%) | 82 (26.0%) | 37 (12.2%) | 119 (19.2%) |
| Insuff. Inform. | 10 (8.3%) | 3 (3.9%) | 13 (6.5%) | 303 (4.2%) | 232 (4.5%) | 535 (4.3%) | 55 (17.4%) | 76 (25.1%) | 131 (21.2%) |
| Surgery | |||||||||
| Yes | 94 (77.7%) | 62 (79.5%) | 156 (78.4%) | 6561 (90.1%) | 4622 (90.3%) | 11,183 (90.2%) | 188 (59.5%) | 181 (59.7%) | 369 (59.6%) |
| No | 27 (22.3%) | 16 (20.5%) | 43 (21.6%) | 718 (9.9%) | 494 (9.7%) | 1,212 (9.8%) | 128 (40.5%) | 122 (40.3%) | 250 (40.4%) |
| Radiation | |||||||||
| Yes | 19 (15.70%) | 24 (30.77%) | 43 (21.6%) | 425 (5.8%) | 357 (7.0%) | 782 (6.3%) | 39 (12.3%) | 33 (10.9%) | 72 (11.6%) |
| No | 102 (84.30%) | 54 (69.23%) | 156 (78.4%) | 6854 (94.2%) | 4759 (93.0%) | 11,613 (93.7%) | 277 (87.7%) | 270 (89.1%) | 547 (88.4%) |
Notes: The percentage figure refers to the total amount of cases in the regional disease category. The table above displays the distribution of clinically relevant disease characteristics for patients with UUT malignancies across the different histologies, ie, transitional cell, squamous cell and others (mainly small cell, sarcomatoid and undifferentiated carcinomas).
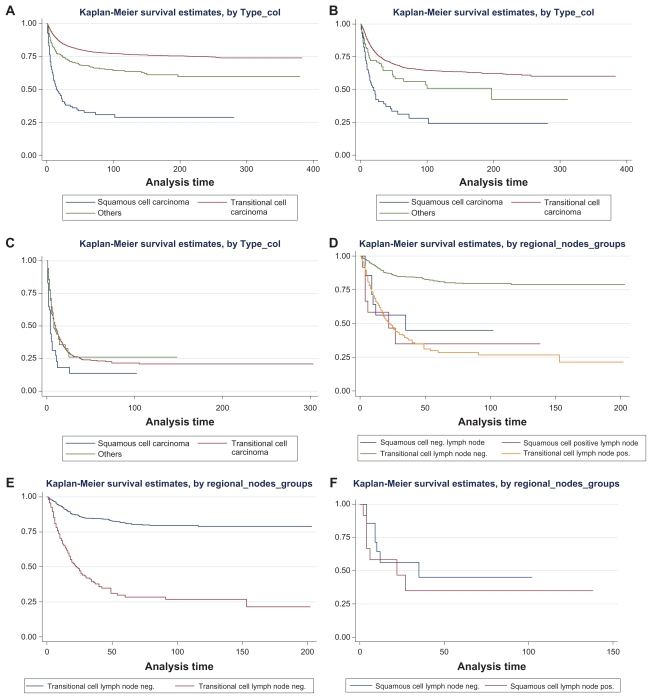
At the time of our analysis approximately 56% of patients with squamous cell malignancies of the upper urothelial tract had died from their underlying malignancies. The death rate from bladder tumors was consistently less than or equal to 1% (2 in the squamous cell group and 93 in the TCC group). The overall survival for patients who presented with UUT squamous cell cancer was significantly worse than for TCC (see Kaplan Meier graph A in graph 1). We retained race, prior surgery and/or radiotherapy, decade of diagnosis, age at diagnosis, gender and stage in our final model. Of note is that the covariate stage was introduced as described above, utilizing the above described staging classification, which collapses extension beyond the organ (renal pelvis or ureter) and local lymph node involvement into regional disease. We performed Cox proportional hazard modeling for cancer specific survival, adjusting for race, prior surgery and radiotherapy, decade of diagnosis, age at diagnosis, gender, preceding malignancies and stage. We then examined the survival difference for regional and metastatic disease across the three different histologies. As graphically illustrated in Kaplan Meier graph C, no significant difference in survival was established for patients presenting with metastatic disease. The major difference in survival is most notable in the patient group presenting with regional stage disease (graph B).
As outlined in Table 1 of the 4,499 patients with regional disease only 684 patients obtained appropriate lymph node sampling (12 with squamous, 648 with transitional cell and 24 with other histological subtype). Graphs D and E of panel 1 demonstrate the survival of patients with loco-regional disease and known lymph node status for TCC (graph D) and SCC(graph E), respectively. In the group of TCC patients with regional and reported lymph node status, survival was significantly decreased in patients with positive lymph nodes (P < 0.01) (graph D). In the small group of patients with SCC no survival difference could be observed in the patients with positive or absent lymph node involvement (P = 0.63) (graph F).
Panel 1.
The panel above graphically illustrates the survival of patients with UUT malignancies. Graph A demonstrates the survival by histology. All stages are considered. Graph B considers only the patients presenting with regional disease, ie, patients with malignancies beyond the confinement of the renal pelvis or ureter or positive lymph node involvement. Graph C displays the survival of patients with metastatic disease, by histology. Graph D illustrates the survival of patients with locoregional disease and known lymph node status. All histologies are included. Graph E demonstrates the superior survival of patients with absent lymph node involvement in patients with transitional cell histology and regional disease status. Graph F demonstrates the absence of a significant difference by lymph node status in patients with regional disease status and squamous cell histology.
Table 2 outlines the fraction of patients with the different histologies still alive at 6, 12, 24 and 60 months after diagnosis. A graphical depiction of these patients’ overall survival estimate is shown in graph A of panel 1. In the upper part of the table we also demonstrate the semi-parametrical survival estimates, displaying hazard ratios, using patients with transitional cell carcinomas as reference group. It clearly illustrates the inferior survival of patients with squamous cell carcinomas, when all presenting patients (all stages) are compared to patients to patients with TCC. Although a trend towards an improved survival is identified in patients with papillary subhistology, when compared with TCC patients, this did not reach statistical significance.
Table 2.
| Sites | HR transitional cell+ | HR squamous cell+ | HR papillary+ | HR other histologies+ |
|---|---|---|---|---|
| Renal pelvis | 1* | 4.30 (95% CI 3.40–5.44) | 0.81 (95% CI 0.47–1.39) | 1.28 (95% CI 1.04–1.58) |
| Ureter | 1* | 3.05 (95% CI 2.11–4.42) | 0.63 (95% CI 0.24–1.69) | 0.99 (95% CI 0.74–1.32) |
| Both sites | 1* | 3.68 (95% CI 3.02–4.50) | 0.77 (95% CI 0.48–1.25) | 1.10 (95% CI 0.93–1.30) |
| Cancer specific survival all stages | ||||
| Six months | 94.0% (95% CI 59.8%–73.9%) | 67.4% (95% CI 59.8%–73.9%) | 97.2% (95% CI 91.8%–99.1%) | 85.6% (95% CI 82.3%–88.3%) |
| One year | 89.7% (95% CI 89.2%–90.3%) | 54.3% (95% CI 46.4%–61.6%) | 93.5% (95% CI 86.9%–96.9%) | 80.0% (95% CI 76.3%–83.3%) |
| Two years | 84.5% (95% CI 83.8%–85.2%) | 40.6% (95% CI 32.7%–38.3%) | 90.4% (95% CI 82.9%–94.7%) | 74.5% (95% CI 70.3%–78.1%) |
| Five years | 79.1% (95% CI 78.2%–79.9%) | 32.5% (95% CI 24.6%–40.6%) | 84.1% (95% CI 74.8%–90.2%) | 68.2% (95% CI 63.5%–72.4%) |
| Cancer specific survival stage IV only | ||||
| Six months | 67.4% (95% CI 64.4%–70.2%) | 40.1% (95% CI 25.8%–54.0%) | NA | 58.3% (95% CI 47.9%–67.4%) |
| One year | 51.9% (95% CI 48.6%–55.1%) | 30.3% (95% CI 16.7%–45.0%) | NA | 45.6% (95% CI 35.0%–55.6%) |
| Two years | 36.9% (95% CI 33.4%–40.4%) | 26.5% (95% CI 13.4%–41.5%) | NA | 31.8% (95% CI 20.9%–43.3%) |
| Five years | 25.9% (95% CI 22.2%–29.7%) | 18.9% (95% CI 7.7%–34.0%) | NA | 20.0% (95% CI 8.8%–34.5%) |
Notes: Reference group;
HR for cancer specific mortality, adjusted for race, prior surgery and radiotherapy, decade of diagnosis, age at diagnosis, gender and stage. The table above describes the survival of patients with UUT malignancies across different histologies. The upper part demonstrates the outcome of the Cox Proportional Hazard model, adjusted for race, prior surgery and radiotherapy, decade of diagnosis, age at diagnosis, gender and stage. Patients with transitional cell histology were considered the reference group. The second part shows the cancer specifc survival for all stages and for patients with stage IV only, respectively. The cancer specific survival for patients with stage IV disease and papillary histology could, secondary to a paucity of cases not be calculated.
Discussion
We present the largest registry based study of squamous cell malignancies of the upper urothelial tract to date. The fraction of SCC in malignancies of the upper urinary tract was 1.5% in our cohort. This is in the range of previous publications which reported the incidence to be 0.7% to 10%.1,8,9,11,16
Most previously published reports regarding the outcome of patients with SCC of the UUT are case reports or small series.6–9 The largest study published to date is a retrospective, registry based study from Sweden.11 The strength of this Swedish study, which included 743 patients with transitional cell and 65 with squamous cell histology, was a central review process of the majority of the specimens and the availability of clinical details from review of hospital records.
Our study reveals several interesting findings. First, SCC of the UUT present on average with more advanced stages when compared to TCCs. This can probably be explained by a more aggressive biology and a lower incidence of clinical warning signs, such as hematuria, in patients with SCC. Second, the overall survival for patients diagnosed in the US with SCC of the UUT is dismal and overall inferior to patients with TCC. Third, stage for stage the survival difference between squamous and transitional histologies is less pronounced. This is in accordance to the prior Swedish study by Holmang et al,3 which demonstrated an equally poor prognosis for both tumor types when only advanced stages were considered. Holmang et al observed a trend towards a decreased survival in SCC that did not reach statistical significance (P = 0.099) in patients with pT3.11 In our study the survival difference at six and twelve months after diagnosis did reach statistical significance. This is largely due to the greater number of observed events thus resulting in a higher statistical power in our study. Fourth, our study demonstrates that patients with TCC involving the regional lymph nodes fare significantly worse than patients with disease extending beyond the organ. This is reflected in the current TNM staging classification17 which considers regional lymph node involvement as stage IV disease. Interestingly, this survival difference is less pronounced in patients with SCC and regional disease. Unfortunately, the paucity of data on patients in our registry with SCC and regional disease of the UUT and inadequate lymph node sampling does not allow for a firm conclusion regarding the prognostic relevance of local lymph node status in patients with SCC of the UUT. Lastly, we did not observe a high association between malignancies of the UUT and death from bladder cancer. Patients in our study only rarely died from bladder cancer (≤1%). This is in contrast to previous reports.18 However, we recognize the high percentage of patients with UUT malignancy that will develop lower urinary tract tumors and the importance of regular lower urinary tract surveillance.
Our study has several shortcomings. First, the SEER registry obtains information from medical charts and, therefore, the registry data can only be as accurate as the medical record from which it is obtained. Second, clinical history and comorbidities cannot be obtained from the SEER registry. This might have introduced a bias of differential attrition across the different histological subtypes into our study. Third, although the SEER registry provides information about postoperative radiotherapy, this has never been shown to be of benefit to patient survival in UUT.19,20 At least three retrospective studies have suggested some benefit from adjuvant chemotherapy.21–23 Unfortunately, the SEER database does not provide data about administered chemotherapy. Lastly, the stage coding on patients with UUT malignancies was largely done in the old AJCC Staging system. This classification collapses local lymph node involvement and tumors exceeding the confinement of the involved organ into the regional stage category. As demonstrated above this leads to inaccurate prognostication, at least in TCCs. Hence, our reported hazard ratio has to be considered with caution.
Despites the shortcomings, the size of this study and the quality of the performed statistical analysis does provide a valuable addition to the existing, but limited, body of literature on squamous cell malignancies of the UUT.
Conclusion
Squamous cell cancers of the upper urinary tract are associated with a poor prognosis. Overall, patients with SCC present with more advanced disease and have lower survival rates when compared to their transitional cell counterparts. Lymph node dissection has not been a standard part of the surgical management of UUT. However, the lymph node status in patients with UUT provides further information to help give patients more accurate prognoses. To improve this prognosis the rigorous future study of effective systemic chemotherapy for palliative and adjuvant treatment, especially in patients with advanced disease, is needed.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Flanigan R. Campbell-Walsh Urology. Saunders; 2007. Urothelial tumors of the upper urinary tract. [Google Scholar]
- 2.Williams CBJM. Carcinoma of the renal pelvis: a review of 43 cases. Br J Urol. 1973;45:370. doi: 10.1111/j.1464-410x.1973.tb12174.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams CBJM. Carcinoma of the ureter—a review of 54 cases. Br J Urol. 1973;45:377. doi: 10.1111/j.1464-410x.1973.tb12175.x. [DOI] [PubMed] [Google Scholar]
- 4.Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164(5):1523–5. [PubMed] [Google Scholar]
- 5.Diaz Gonzalez R, et al. Squamous cell carcinoma of the renal pelvis associated with hypercalcemia and the presence of parathyroid hormone-like substances in the tumor. J Urol. 1985;133(6):1029–30. doi: 10.1016/s0022-5347(17)49364-5. [DOI] [PubMed] [Google Scholar]
- 6.Kazarians B, et al. Squamous cell carcinoma of the renal pelvis. Therapeutic options for a rare entity. Aktuelle Urol. 2008;39(6):456–8. doi: 10.1055/s-2008-1038233. [DOI] [PubMed] [Google Scholar]
- 7.Washino S, et al. Two cases of squamous cell carcinoma of upper urinary tract with hypercalcemia. Nippon Hinyokika Gakkai Zasshi. 2008;99(6):703–8. doi: 10.5980/jpnjurol1989.99.703. [DOI] [PubMed] [Google Scholar]
- 8.Busby JE, et al. Upper urinary tract tumors with nontransitional histology: a single-center experience. Urology. 2006;67(3):518–23. doi: 10.1016/j.urology.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Li MK, Cheung WL. Squamous cell carcinoma of the renal pelvis. J Urol. 1987;138(2):269–71. doi: 10.1016/s0022-5347(17)43116-8. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi E, et al. Lymphovascular invasion independently predicts increased disease specific survival in patients with transitional cell carcinoma of the upper urinary tract. J Urol. 2005;174(6):2120–3. doi: 10.1097/01.ju.0000181801.22474.8b. discussion 2124. [DOI] [PubMed] [Google Scholar]
- 11.Holmang S, Lele SM, Johansson SL. Squamous cell carcinoma of the renal pelvis and ureter: incidence, symptoms, treatment and outcome. J Urol. 2007;178(1):51–6. doi: 10.1016/j.juro.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Edition T, editor. International Classification of Diseases in Oncology. World Health Organization; Geneva: WHO; 2000. [Google Scholar]
- 13.Cox D. Regression models and life tables. J R Stat Soc B. 1972;26:187–220. [Google Scholar]
- 14.Grambsch PMTT. Proportional hazard tests and diagnostics based on weighted residuals. Biometrica. 1994;81:515–26. [Google Scholar]
- 15.Schoenfeld Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–41. [Google Scholar]
- 16.Brown G, Matin SF. Busby Ability of clinical grade to predict final pathologic stage in upper urinary tract transitional cell carcinoma: implications for therapy. Urology. 2007;252 doi: 10.1016/j.urology.2007.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edition S, editor. AJCC Cancer Staging Manual. New York: Springer-Verlag; 2002. [Google Scholar]
- 18.Korkes F, et al. Carcinoma of the renal pelvis and ureter. Int Braz J Urol. 2006;32(6):648–53. doi: 10.1590/s1677-55382006000600005. discussion 653–5. [DOI] [PubMed] [Google Scholar]
- 19.Cozad SC, et al. Transitional cell carcinoma of the renal pelvis or ureter: patterns of failure. Urology. 1995;46(6):796–800. doi: 10.1016/S0090-4295(99)80346-X. [DOI] [PubMed] [Google Scholar]
- 20.Cozad SC, et al. Adjuvant radiotherapy in high stage transitional cell carcinoma of the renal pelvis and ureter. Int J Radiat Oncol Biol Phys. 1992;24(4):743–5. doi: 10.1016/0360-3016(92)90723-u. [DOI] [PubMed] [Google Scholar]
- 21.Kwak C, et al. Adjuvant systemic chemotherapy in the treatment of patients with invasive transitional cell carcinoma of the upper urinary tract. Urology. 2006;68(1):53–7. doi: 10.1016/j.urology.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 22.Michael M, et al. Adjuvant chemotherapy for high-risk urothelial transitional cell carcinoma: the Princess Margaret Hospital experience. Br J Urol. 1998;82(3):366–72. doi: 10.1046/j.1464-410x.1998.00746.x. [DOI] [PubMed] [Google Scholar]
- 23.Bamias A, et al. Adjuvant chemotherapy with paclitaxel and carboplatin in patients with advanced carcinoma of the upper urinary tract: a study by the Hellenic Cooperative Oncology Group. J Clin Oncol. 2004;22(11):2150–4. doi: 10.1200/JCO.2004.09.043. [DOI] [PubMed] [Google Scholar]