Abstract
Increasing evidence suggests that the aryl hydrocarbon receptor (AhR) pathway has an important role in the regulation of inflammatory responses. Most recently, we have shown that the activation of the AhR pathway by a potent AhR agonist inhibits the development of dextran sodium sulfate (DSS)-induced colitis, a model of human ulcerative colitis, by the induction of prostaglandin E2 (PGE2) in the large intestine. Because several strains of probiotic lactic acid bacteria have been reported to inhibit DSS-induced colitis by unidentified mechanisms, we hypothesized that particular strains of lactic acid bacterium might have the potential to activate the AhR pathway, thereby inhibiting DSS-induced colitis. This study investigated whether there are specific lactic acid bacterial strains that can activate the AhR pathway, and if so, whether this AhR-activating potential is associated with suppression of DSS-induced colitis. By using AhR signaling reporter cells, we found that Lactobacillus bulgaricus OLL1181 had the potential to activate the AhR pathway. OLL1181 also induced the mRNA expression of cytochrome P450 family 1A1 (CYP1A1), a target gene of the AhR pathway, in human colon cells, which was inhibited by the addition of an AhR antagonist, α-naphthoflavon (αNF). In addition, mice treated orally with OLL1181 showed an increase in CYP1A1 mRNA expression in the large intestine and amelioration of DSS-induced colitis. Thus, OLL1181 can induce activation of the intestinal AhR pathway and inhibit DSS-induced colitis in mice. This strain of lactic acid bacterium has therefore the potential to activate the AhR pathway, which may be able to suppress colitis.
Keywords: aryl hydrocarbon receptor, colitis, lactic acid bacterium, probiotics
Aryl hydrocarbon receptor (AhR) recognizes numerous small xenobiotic and natural molecules, such as dioxin and various naturally occurring chemicals, and is involved in the metabolism of these compounds.1 Upon ligand binding, AhR in the cytosol translocates to the nucleus, dimerizes with the AhR nuclear translocator, interacts with the xenobiotic-responsive element (XRE) in the promoter regions of target genes, and initiates the transcription of target genes, such as the xenobiotic-metabolizing enzyme cytochrome P450 1A1 (CYP1A1).1
AhR was first discovered as a mediator of dioxin toxicity, but the AhR pathway is now thought to have diverse physiological roles in many different cellular functions.2, 3 Recent studies also highlight AhR as an important regulator of inflammation and immunity.4, 5 For instance, AhR inhibits the lipopolysaccharides signaling pathway through an interaction with Stat1 in macrophages,6 suggesting that AhR negatively regulates the lipopolysaccharides-induced inflammatory response.
Dextran sodium sulfate (DSS)-induced colitis in rodents is characterized by epithelial disruption, resulting in luminal bacterial translocation and subsequent infiltration of neutrophils and other acute immune cells.7 These features recapitulate the events that lead to acute mucosal injury of human ulcerative colitis.7, 8 We have previously shown that activation of the AhR pathway inhibits DSS-induced colitis in mice, possibly through induction of prostaglandin E2 (PGE2) in the large intestine.9 These findings have prompted us to hypothesize that environmental factors that can ameliorate DSS-induced colitis might do so by activating the AhR pathway.
Probiotics, live commensal microorganisms that are beneficial to human and animal health, have been shown to be effective for preventing or treating various diseases, including inflammatory bowel diseases.10, 11 Such health-promoting effects have been attributed to either the beneficial effects on intestinal microbiota or to the immunoregulatory effects of microbial components and metabolites, or both.12 Previous studies have reported that several probiotic lactic acid bacterial strains can inhibit DSS-induced colitis in rodents,13, 14, 15 although the precise mechanisms remain unclear.
The present study investigated whether there are specific lactic acid bacterial strains that can activate the AhR pathway, and if so, whether this AhR-activating potential is associated with suppression of DSS-induced colitis. We have previously established a specific and sensitive in vitro bioassay for screening AhR-activating compounds in environmental samples.16 Using this bioassay system, we screened a lactic acid bacterial library kept at our facility for their AhR pathway-activating potential, and then investigated whether such lactic acid bacterial strains can inhibit DSS-induced colitis in mice.
RESULTS
Screening of lactic acid bacterial strains for stimulatory effects on the AhR pathway
We screened 62 heat-killed lactic acid bacterial strains maintained by our facility for their potential to activate the AhR pathway using an in vitro bioassay system that we established previously.16 In brief, a murine hepatoma cell line (Hepa-1c1c7) was stably transfected with a secreted alkaline phosphatase (SEAP) gene under the control of the XRE consensus sequences. The established sensor clone HeXS34 secreted SEAP following stimulation with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (positive control) or other chemicals that can activate the AhR pathway. We found that 47 out of 62 (about 75%) heat-killed lactic acid bacterial strains, including L. bulgaricus OLL1181 (see below), significantly increased SEAP activity over 2-folds in the HeXS34 reporter cells (Supplementary Figure 1). Heat-killed, but not live, lactic acid bacterial strains were used in this study because proliferation of live bacteria can affect the bioassay, thereby making the data from the bioassay not consistent.
L. bulgaricus OLL1181 can activate the AhR pathway in vitro and in vivo
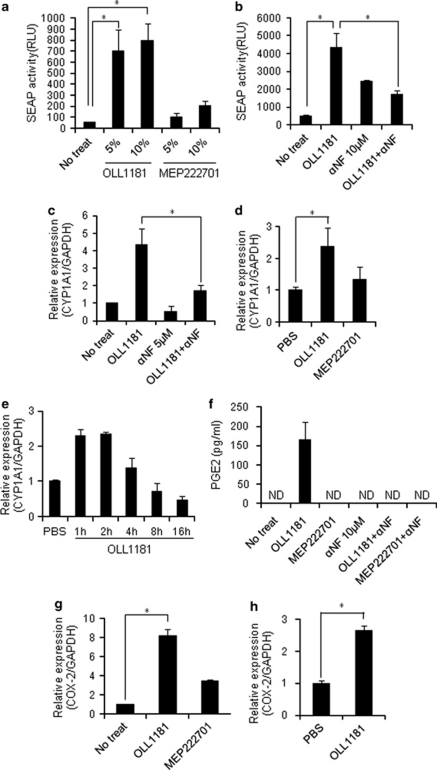
Because L. bulgaricus was one of the lactic acid bacteria, commonly consumed as part of fermented foods, with specially added active live cultures such as in yogurt, we focused on L. bulgaricus OLL1181 strain for further experiments, and used Lactobacills gasseri MEP222701 (which induced minimal SEAP activity in HeXS34 cells) as a negative control. OLL1181, but not MEP222701, increased the SEAP activity in HeXS34 cells, and this increase in activity was inhibited by the addition of an AhR antagonist α-naphthoflavone (αNF) (Figures 1a and b). In the human colonic epithelial cell line, Caco2, OLL1181 also significantly induced CYP1A1 mRNA expression, which was again inhibited by the addition of αNF (Figure 1c). In addition, mice treated orally with one single-dose of OLL1181, but not MEP222701, demonstrated the induction of CYP1A1 mRNA expression in the large intestine (Figure 1d). The increase in CYP1A1 mRNA in the large intestine showed peak at 1–2 h and gradually lost thereafter following the treatment with OLL1181 (Figure 1e). These results suggest that OLL1181, but not MEP222701, can activate the intestinal AhR pathway both in vitro and in vivo.
Figure 1.
Lactobacillus bulgaricus OLL1181 induces activation of the AhR/XRE pathway both in vitro and in vivo. (a) HeXS34 cells were stimulated with suspensions of heat-killed OLL1181 or MEP222701 (5 or 10%) for 24 h. The activity of SEAP in the culture medium was evaluated by chemiluminescent assay. (b) HeXS34 cells pretreated with or without a partial antagonist of the AhR, α-naphthoflavone (αNF; 10 μ) were stimulated with suspensions of heat-killed OLL1181 (10%) for 24 h. The activity of SEAP in the culture medium was evaluated by chemiluminescent assay. (c) Caco2 cells pretreated with or without 5 μ αNF were stimulated with suspensions of heat-killed OLL1181 or MEP222701 (10%) for 4 h. Real-time PCR was performed for detection of CYP1A1 mRNA. (d) Mice were fed 200 μl suspensions of heat-killed OLL1181, MEP222701 or phosphate-buffered saline. At 4 h later, the large intestine of each mouse was resected, and the mRNA expression level of CYP1A1 was examined by real-time PCR. (e) Mice were fed 200 μl suspensions of heat-killed OLL1181 or PBS. The large intestine of each mouse was resected at the indicated times following the treatment and the mRNA expression level of CYP1A1 was examined by real-time PCR. (f) Caco2 cells pretreated with or without 10 μ αNF were stimulated with suspensions of heat-killed OLL1181 or MEP222701 for 24 h. The PGE2 levels in the culture supernatants were measured by enzyme-linked immunosorbent assay. (g) Caco2 cells were stimulated with suspensions of heat-killed OLL1181 or MEP222701 (10%) for 4 h, then real-time PCR was performed to detect COX2 mRNA. (h) Mice were fed 200 μl suspensions of heat-killed OLL1181or phosphate-buffered saline. At 4 h later, the large intestine of each mouse was resected, and the mRNA expression of COX2 was examined by real-time PCR. The values represent the means±s.d. (n=3 per group). *P<0.05 compared with the corresponding controls. Similar results were obtained from two other independent experiments (a–g).
L. bulgaricus OLL1181 increases PGE2 production by Caco2 cells
Because TCDD, a potent AhR activator, induced PGE2 production in Caco2 cells,9 we examined the effects of OLL1181 on PGE2 production in Caco2 cells. OLL1181, but not MEP222701, induced PGE2 production in Caco2 cells, which was inhibited by the addition of αNF (Figure 1f). We also found that OLL1181, but not MEP222701, induced the mRNA expression of cyclooxgenase 2 (COX2), which is a key enzyme involved in PGE2 production (Figure 1g).17 In addition, mice treated orally with OLL1181 showed an increase in COX2 mRNA expression in the large intestine (Figure 1h). These results suggest that OLL1181 induced PGE2 production in Caco2 cells in an AhR pathway-dependent manner, which was associated with increased COX2 expression.
L. bulgaricus OLL1181 ameliorates acute DSS-induced colitis in mice
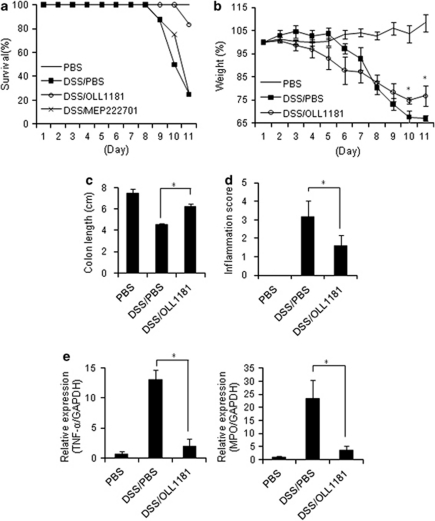
Finally, we examined the effects of OLL1181 on the development of DSS-induced colitis in mice. The mice administered DSS that received a vehicle control orally for 7 days exhibited an 80% mortality rate by day 11 after DSS administration. The MEP222701-treated mice also had an 80% mortality rate at 11 days after DSS. Conversely, OLL1181-treated mice showed a marked increase in survival, with a 20% mortality rate on day 11 after the administration of DSS (Figure 2a). In addition, OLL1181 prevented DSS-induced colitis in mice, as indicated by reduced body weight loss, increased colon length, inflammatory scores and reduced tumor necrosis factor-α and myeloperoxidase expression in the large intestine compared with the control (Figures 2b–e). These results suggest that OLL1181 can ameliorate DSS-induced colitis in mice.
Figure 2.
Lactobacillus bulgaricus OLL1181 ameliorates acute DSS-induced colitis in mice. Mice administered a 3% DSS solution in drinking water for 7 days (days 1–8), followed by regular drinking water for an additional 3 days (days 8–11), were treated with phosphate-buffered saline, MEP222701, and OLL1181 as described in the text. (a) Survival was monitored until day 11 after the start of DSS (n=10–12 mice per group). (b) Body weight changes in mice after the start of DSS (n=4 mice per group). (c) Colon length in mice on day 11 (n=4 mice per group). (d) Inflammation scores in mice on day 11 (n=4 mice per group). (e) Real-time PCR for tumor necrosis factor-α and myeloperoxidase expression in the colon on day 11 (n=4 mice per group). The values represent the means±s.d. *P<0.05 compared with the corresponding controls. Similar results were obtained from two other independent experiments (b–e).
DISCUSSION
We first screened a lactic acid bacterial library, kept at our facility, for their AhR pathway-activating potential by the bioassay system we have established before.16 The reliability of the bioassay to detect xenobiotic AhR ligands has been extensively tested.16, 18, 19 This bioassay achieves linear and dose-dependent responses, exclusively to agonists of the AhR. Our observation that the OLL1181-induced increase in CYP1A1 mRNA expression was inhibited by an AhR antagonist (αNF) also supports that OLL1181 has specific AhR-activating potential.
The components of OLL1181 that activate the AhR pathway remain to be determined. Our screening data showed that about 75% of lactic acid bacterial strains tested here have AhR-activating potential, suggesting that the effective components are relatively conserved across the lactic acid bacterial strains. AhR ligands have been generally classified into two categories, synthetic or naturally occurring.20 The first ligands to be discovered were synthetic halogenated aromatic hydrocarbons (dibenzodioxins and so on) and polycyclic aromatic hydrocarbons (3-methylcholanthrene, benzo(a)pyrene and so on), giving the receptor its name. Naturally occurring compounds that have been identified as ligands of the AhR include derivatives of tryptophan and arachidonic acid metabolites.21, 22 Thus, it is possible that such naturally occurring metabolites derived from OLL1181 or other probiotic strains are responsible for their AhR-activating potential.
We previously showed that an artificial AhR agonist, TCDD, induced PGE2 production in the mouse large intestine.9 However, in the current study, we failed to show increased PGE2 production in the large intestine of mice treated orally with OLL1181, (data not shown) although we showed that there was an increase in the mRNA expression of COX2, an enzyme involved in PGE2 production. The differences in the results might be because of the different AhR-agonistic activities of the two substances. TCDD has a very strong AhR-activating potential with a half-life of 2 weeks in mice, whereas OLL1181 has much lower AhR-activating potential compared with TCDD, and its half-life in mice is unknown. We thus speculate that OLL1181 might induce a weak PGE2 production in the mouse large intestine (through the AhR pathway) that is under the threshold of enzyme-linked immunosorbent assay detection, which can be attributed to the protection against DSS-induced colitis.
We found that one single-dose of oral OLL1181 treatment transiently increased CYP1A1 mRNA in the mouse colon (Figure 1e) and failed to inhibit DSS-induced colitis (Supplementary Figure 2). These findings suggest that persistent, although weak, activation of the intestinal AhR pathway obtained by daily administration of OLL1181 might be necessary for the protection against DSS-induced colitis.
OLL1181-treated mice showed a tendency to reduce body weight more at days 4–6 during the DSS treatment than phosphate-buffered saline-treated mice, although it was not statistically significant (Figure 2b). A previous study suggested that TCDD exposure was associated with decreased survival, neutrophilia and elevated interferon-γ levels in the lungs following influenza virus infection,23 suggesting enhanced innate immune response by AhR activation. Similar inflammatory events might occur at early time points in our DSS-induced colitis model, but prolonged AhR activation by daily OLL1181 treatment might increase production of protective factors against intestinal injury such as PGE2 at later time points, thereby ameliorating colitis. However, the precise mechanism(s) by which OLL1181 ameliorates DSS-induced colitis in mice remains to be investigated.
In summary, we identified L. bulgaricus OLL1181 as a probiotic bacterial strain that can activate the AhR pathway, leading to suppression of experimental colitis. To our knowledge, this is the first study to demonstrate that a particular lactic acid bacterial strain has AhR-activating potential, and that this property might be linked to the probiotic effects of lactobacilli.
METHODS
Microorganisms
Lactic acid bacterial strains maintained by our facility (Meiji Dairies, Kanagawa, Japan) were cultured in Lactobacilli MRS broth (Becton Dickinson, Sparks, MD, USA) at 37 °C for 18 h. After fermentation, the cells were collected in a refrigerated centrifuge (10 000 g, 15 min) and washed twice with a saline solution, followed by a wash with water. The cells were re-suspended in distilled water (the cell counts were 5 × 109 colony forming units (CFU) ml−1, based on a conventional assay on MRS broth plates containing 1.5% agar), heat-killed at 75 °C for 60 min, and lyophilized.
Screening assay for AhR pathway activation
HeXS34 (Hepa-1c1c7–derived, pXRE-SEAP-transfected clone no. 34) cells were established by transfection of Hepa-1c1c7 cells with pXRE-SEAP, which introduces a SEAP gene under the control of two copies of the XRE consensus sequence.16 The cells were maintained in MEMα (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum. The reporter cells (5000/90 μl/well) were seeded in 96-well plates and cultured for 24 h in the absence (control) or in the presence of 10 μl lyophilized heat-killed lactic acid bacterium strains, unless specifically stated. The culture media of the reporter cells were then subjected to chemiluminescent assay to evaluate SEAP activity. To examine the role of the AhR in the activation of XRE, HeXS34 cells were either left untreated, or were pretreated with the indicated doses of αNF (an AhR antagonist) for 30 min. The media was then replaced with fresh media, and then cells were exposed to heat-killed lactic acid bacterial strains. As a positive control, cells were treated with 50 pM TCDD.
SEAP assay
Activity of SEAP in conditioned media was evaluated by a chemiluminescent method using the Great EscAPe SEAP detection kit (Clontech, Carlsbad, CA, USA) as previously described.16 Assays were performed in triplicate unless specifically stated otherwise.
Cell culture
The human Caco2 colonic adenocarcinoma cells were cultured in DMEM (Invitrogen/Gibco, Mountain View, CA, USA) supplemented with 10% fetal bovine serum in the presence of antibiotics at 37 °C in a humidified atmosphere in the presence of 5% CO2.
Quantitative real-time PCR
Quantitative PCR analysis of cDNA was performed using an ABI 7300 real-time PCR system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions using primers and probes for mouse or human CYP1A1, COX2, tumor necrosis factor-α, myeloperoxidase and glyceraldehyde-3-phosphate dehydrogenase (Applied Biosystems) as previously described.24 The ratio of each gene to that of glyceraldehyde-3-phosphate dehydrogenase was calculated, and the relative expression levels are shown.
PGE2 enzyme-linked immunosorbent assay
The amounts of secreted PGE2 were determined using a PGE2 Competitive ELISA kit (Thermo Scientific, Waltham, MA, USA) according to the manufacturer's instructions.
Induction of colitis
Female 4 to 6-week-old C57BL/6 mice were purchased from Japan SLC (Tokyo, Japan) and were bred under specific pathogen-free conditions. To induce colitis, mice were treated daily with 3% DSS (MW, 5000; Wako Pure Chemical, Osaka, Japan) dissolved in distilled drinking water for 7 days, followed by distilled water (no DSS) for 3 days, as previously described.9, 25 To assess the preventive effects of the bacterial strains, the mice were fed daily with a 200 μl suspension (1 × 109 CFU) of heat-killed lactic acid bacteria (OLL1181 and MEP222701) for the first 7 days by gastric lavage. Control mice were fed 200 μl phosphate-buffered saline daily for the first 7 days. For some experiments, mice were fed a 200 μl suspension (1 × 109 CFU) of heat-killed OLL1181 or phosphate-buffered saline without DSS administration. The degree of inflammation was histologically scored as previously described.25 Briefly, scores of inflammation were: 0, no increased inflammatory infiltrates; 1, focal mild inflammation; 2, diffuse mild inflammation; 3, cryptic abscess formation; 4, diffuse dense inflammation. All animal experiments were approved by the Institutional Review Board of the University of Yamanashi.
Statistical analysis
The values represent the means±s.d. The statistical analysis was performed using unpaired Student's t-tests. A value of P<0.05 was considered to be significant.
Acknowledgments
We thank Ms Yuko Ohnuma, Mutsuko Hara, and Dr Shuji Matsuoka for their valuable assistance. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors received grant support from Meiji Dairies.
Footnotes
Supplementary Information accompanies the paper on Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007;581:3608–3615. doi: 10.1016/j.febslet.2007.03.046. [DOI] [PubMed] [Google Scholar]
- Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol. 2009;77:713–722. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30:447–454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Pizarro TT, Arseneau KO, Bamias G, Cominelli F. Mouse models for the study of Crohn's disease. Trends Mol Med. 2003;9:218–222. doi: 10.1016/s1471-4914(03)00052-2. [DOI] [PubMed] [Google Scholar]
- Takamura T, Harama D, Matsuoka S, Shimokawa N, Nakamura Y, Okumura K, et al. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol Cell Biol. 2010;88:685–689. doi: 10.1038/icb.2010.35. [DOI] [PubMed] [Google Scholar]
- Kopp-Hoolihan L. Prophylactic and therapeutic uses of probiotics: a review. J Am Diet Assoc. 2001;101:229–238. doi: 10.1016/S0002-8223(01)00060-8. [DOI] [PubMed] [Google Scholar]
- Sanders ME. Probiotics: considerations for human health. Nutrition Rev. 2003;61:91–99. doi: 10.1301/nr.2003.marr.91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross ML, Gill HS. Can immunoregulatory lactic acid bacteria be used as dietary supplements to limit allergies. Int Arch Allergy Immunol. 2001;125:112–119. doi: 10.1159/000053804. [DOI] [PubMed] [Google Scholar]
- Herías MV, Koninkx JF, Vos JG, Huis in't Veld JH, van Dijk JE. Probiotic effects of Lactobacilluscasei on DSS-induced ulcerative colitis in mice. Int J Food Microbiol. 2005;103:143–155. doi: 10.1016/j.ijfoodmicro.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Osman N, Adawi D, Molin G, Ahrne S, Berggren A, Jeppsson B. Bifidobacterium infantis strains with and without a combination of oligofructose and inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMC Gastroenterol. 2006;6:31. doi: 10.1186/1471-230X-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Han SY, Bae EA, Huh CS, Ahn YT, Lee JH, et al. Lactic acid bacteria inhibit proinflammatory cytokine expression and bacterial glycosaminoglycan degradation activity in dextran sulfate sodium-induced colitic mice. Int Immunopharmacol. 2008;8:574–580. doi: 10.1016/j.intimp.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Kasai A, Hiramatsu N, Meng Y, Yao J, Takeda M, Maeda S, et al. DRESSA: biosensing of dioxin and dioxin-like chemicals using secreted alkaline phosphatase. Anal Biochem. 2004;335:73–80. doi: 10.1016/j.ab.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Santovito D, Mezzetti A, Cipollone F. Cyclooxygenase and prostaglandin synthases: roles in plaque stability and instability in humans. Curr Opin Lipidol. 2009;20:402–408. doi: 10.1097/MOL.0b013e32832fa22c. [DOI] [PubMed] [Google Scholar]
- Kasai A, Hiramatsu N, Meng Y, Yao J, Maeda S, Kitamura M. Fast-track DRESSA: a bioassay for fast, sensitive, and selective detection of halogenated and polycyclic aromatic hydrocarbons. Anal Biochem. 2005;337:84–88. doi: 10.1016/j.ab.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Kasai A, Yao J, Yamauchi K, Hiramatsu N, Hayakawa K, Meng Y, et al. Influence of cAMP on reporter bioassays for dioxin and dioxin-like compounds. Toxicol Appl Pharmacol. 2006;211:11–19. doi: 10.1016/j.taap.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Adachi J, Mori Y, Matsui S, Takigami H, Fujino J, Kitagawa H, et al. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem. 2001;276:31475–31478. doi: 10.1074/jbc.C100238200. [DOI] [PubMed] [Google Scholar]
- Seidel SD, Winters GM, Rogers WJ, Ziccardi MH, Li V, Keser B, et al. Activation of the Ah receptor signaling pathway by prostaglandins. J Biochem Mol Toxicol. 2001;15:187–196. doi: 10.1002/jbt.16. [DOI] [PubMed] [Google Scholar]
- Neff-LaFord H, Teske S, Bushnell TP, Lawrence BP. Aryl hydrocarbon receptor activation during influenza virus infection unveils a novel pathway of IFN-γ production by phagocytic cells. J Immunol. 2007;179:247–255. doi: 10.4049/jimmunol.179.1.247. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol. 2008;122:1208–1214. doi: 10.1016/j.jaci.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Hahm KB, Im YH, Parks TW, Park SH, Markowitz S, Jung HY, et al. Loss of transforming growth factor-β signalling in the intestine contributes to tissue injury in inflammatory bowel disease. Gut. 2001;49:190–198. doi: 10.1136/gut.49.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.