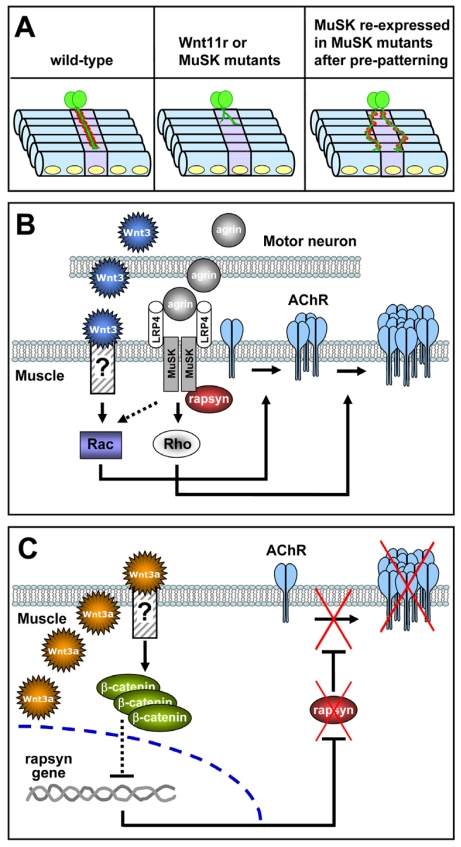
Figure 1.
Wnt ligands modulate postsynaptic differentiation at the vertebrate NMJ. (A) Wnts induce aneural AChR clustering. In zebrafish, Wnt11r silencing results in strong axonal branching defects associated with impaired AChR pre-patterning, similar to MuSK null mutants (middle panel). MuSK rescue after pre-patterning inhibition (induced by MuSK depletion), resulted in mislocalized, but still functional, neuromuscular synapses (right panel); (B) Wnts as positive cues for postsynaptic differentiation. Wnt3 and agrin released from motoneurons collaborate to promote the formation of AChR clusters. Wnt3-induced AChR microclusters via Rac1 are converted into large clusters by agrin, which promotes the further activation of Rac1 and Rho; (C) Wnt-dependent disaggregation of AChR clusters. Wnt3a, secreted by muscle cells at the stages of NMJ formation, activates a β-catenin pathway that induces the dispersal of AChR clusters through the inhibition of rapsyn expression.