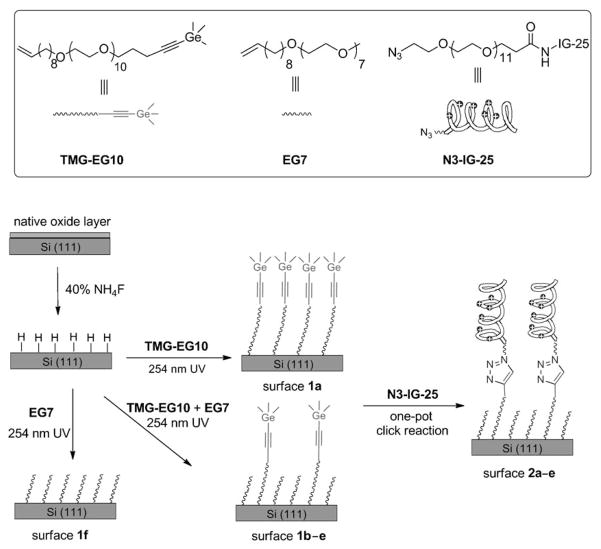
Scheme 1.
Surface hydrosilylation using a mixture of the TMG-protected alkenyne TMG-EG10 and the alkene EG7 on H-terminated Si(111) surfaces, followed by attachment of IG-25 with an azido tag (N3-IG-25). The mole fraction of TMG-EG10 was 1.0, 0.4, 0.2, 0.1, 0.05 or 0, to afford the corresponding surface 1a–f, respectively. The corresponding peptide-presenting surfaces 2a–e are formed upon CuAAC reaction of N3-IG-25 with the TMG-alkyne surfaces 1a–e.