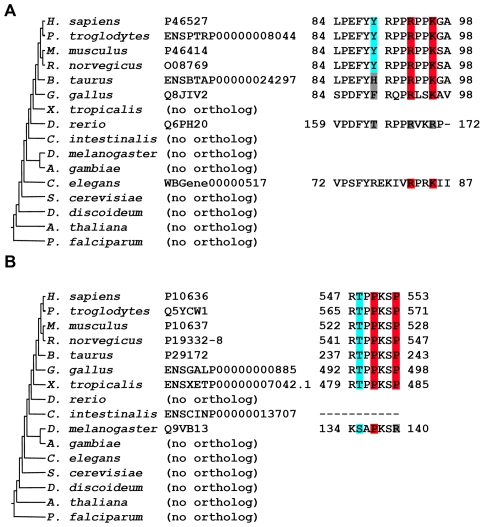
Figure 4. Step-wise appearance of motifs and potential phosphorylation sites.
(A) The motif is older than the potential phosphorylation site. The human CDK inhibitor 1B (top line) includes an SH3-binding motif (RxxK, highlighted in red) and a proximal tyrosine that may affect the motif's interaction potential upon phosphorylation [74], [75] (highlighted in cyan). The sequence pattern is conserved from C. elegans to human, but the tyrosine is conserved only between rat and human. This suggests that an old domain-binding motif has gained phospho-regulation in more recent organisms. Protein accessions are according to the Uniprot or Ensembl databases. (B) Potential phosphorylation site is older than the motif. The human Tau protein includes an SH3-binding motif (PxxP) and a proximal threonine that inhibits the motif's interaction potential upon phosphorylation [76]. This phosphorylation was also shown to induce a conformational change that unlocks the closed form of the protein [77]. The motif is conserved from X. tropicalis to human, while the potential phosphorylation site may have appeared earlier in evolution (present in D. melanogaster). This suggests that the domain-binding potential was established close to already functional phosphorylation.